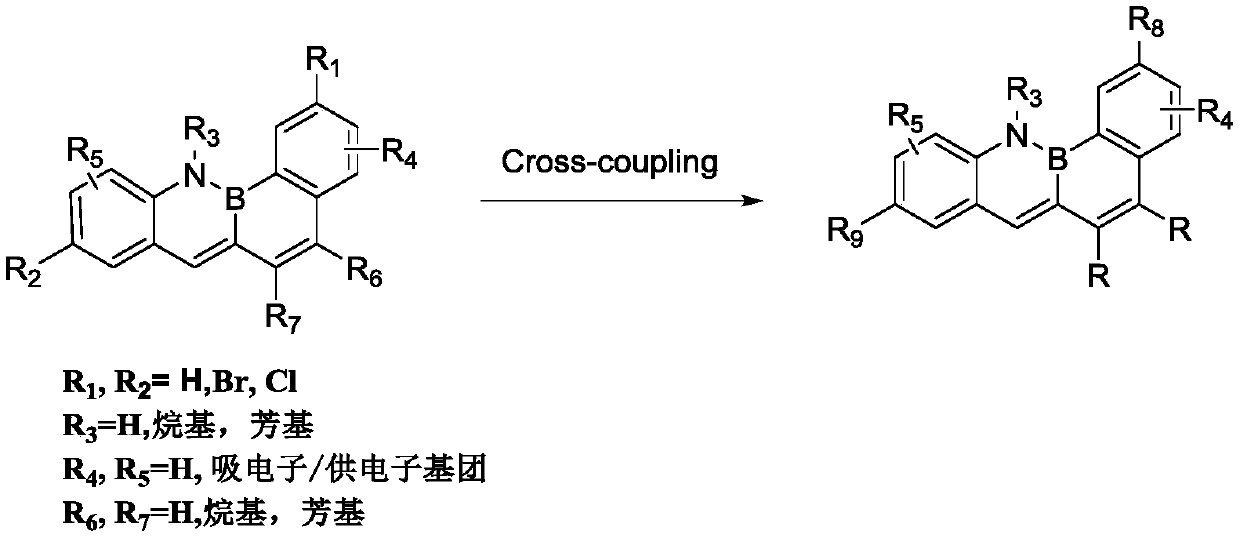
Synthesis method of boron-nitrogen benzanthracene fused-ring compound
A synthesis method, the technology of benzanthracene, applied in the field of organic synthesis, can solve the problems of poor selectivity, achieve the effects of less environmental pollution, strong reaction specificity, and avoid the use of sensitive reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
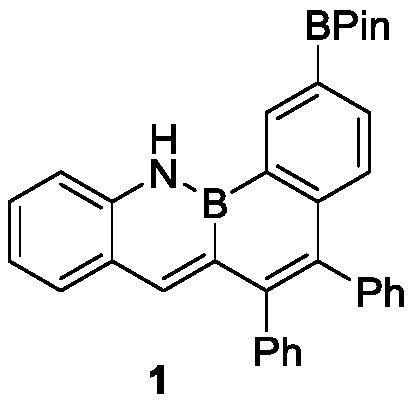
[0020] Synthesis of compound 1: take a 50mL dry Schlenk bottle, vacuumize and change the argon for 3 times, under the protection of argon, add monobromoborazanthracene (230mg, 0.5mmol), B2Pin2 (150mg, 0.75mmol), Pd( dppf)Cl2 (6mg, 2mol%), Na2CO3 (80mg, 0.75mmol), and introduce anhydrous and oxygen-free solvent tetrahydrofuran (5mL). Under the protection of argon, react at 60°C for about 16h. After the reaction is completed, after the temperature of the system drops to room temperature, extract with dichloromethane (4*10mL) and water (50mL), combine the organic phases, dry them with anhydrous sodium sulfate, and reduce pressure The solvent was removed and separated by column chromatography (developing solvent: n-hexane:dichloromethane=5:1) to obtain 170 mg of a yellow-green solid with a yield of 67%.
[0021] The nuclear magnetic analysis data of this compound are as follows:
[0022] 1H NMR (400MHz, CDCl3): δ9.18 (1H, s, NH), 8.89 (1H, s), 7.94 (1H, dd, J1 = 4Hz, J2 = 8Hz), 7...
Embodiment 2
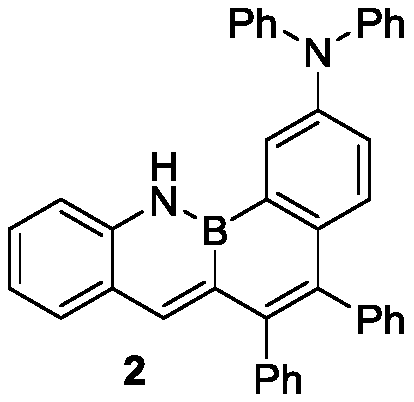
[0025] Synthesis of compound 2: Take a 50mL dry Schlenk bottle, vacuumize and change argon three times, under the protection of argon, add monobromoborazanthracene (230mg, 0.5mmol), diphenylamine (102mg, 0.6mmol), alkene Propylpalladium chloride dimer (2mg, 1mol%), JohnPhos ligand ((3mg, 2mol%), NaOt-Bu (67mg, 0.7mmol), import anhydrous oxygen-free solvent toluene (4mL). Argon protection At 80°C for about 16 hours, the reaction was completed. After the temperature of the system dropped to room temperature, the solid insolubles were removed by filtration, washed with dichloromethane, and the solvent was removed under reduced pressure. 5:1), 227 mg of yellow solid was obtained, and the yield was 83%.
[0026] The nuclear magnetic analysis data of this compound are as follows:
[0027] 1 H NMR (400MHz, CDCl 3 ):δ8.81(1H,s,NH),8.18(1H,s),7.99(1H,s),7.69(1H,d,J=8Hz),7.50-7.58(2H,m),7.11-7.30 (21H,m),7.02-7.08(2H,m). 11 B NMR (128MHz, CDCl 3 ): δ31.36. 13 C NMR (101MHz, CDCl ...
Embodiment 3
[0030] Synthesis of compound 3: take a 50mL dry Schlenk bottle, vacuumize and change argon three times, under the protection of argon, add dibromoborazanthracene (270mg, 0.5mmol), diphenylamine (204mg, 1.2mmol), diphenylamine (204mg, 1.2mmol), ene Propylpalladium chloride dimer (4mg, 2mol%), JohnPhos ligand (6mg, 4mol%), NaOt-Bu (134mg, 1.4mmol) were introduced into anhydrous and oxygen-free solvent toluene (10mL). Under the protection of argon, react at 80°C for about 16h. After the reaction is completed, when the temperature of the system drops to room temperature, remove the solid insoluble matter by filtration, wash with dichloromethane, remove the solvent under reduced pressure, and separate by column chromatography (developing agent: n-hexane: two Chloromethane=5:1), to obtain 261 mg of yellow-green solid, yield 73%.
[0031] The nuclear magnetic analysis data of this compound are as follows:
[0032] 1 H NMR (400MHz, CDCl 3 ):δ8.79(1H,s,NH),8.16(1H,d,J=4Hz),7.79(1H,s),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com