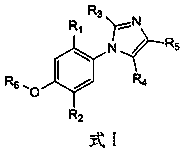
Phenylimidazole derivative, synthesis method of phenylimidazole derivative and application of phenylimidazole derivative to pesticide
The technology of a phenylimidazole and a synthesis method is applied in the field of pesticides and achieves the effects of good bactericidal effect, simple structure and easy synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
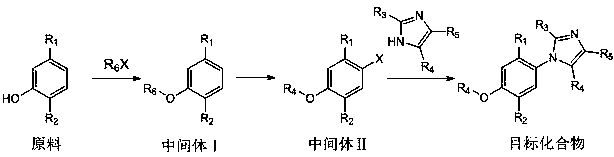
[0036] Its synthetic steps of the synthetic method of phenylimidazole derivative of the present invention are as follows:
[0037] .
[0038] The application of the phenylimidazole derivatives of the present invention in pesticides is to inhibit or kill phytopathogenic fungi, and the phytopathogenic fungi that are inhibited or killed are tomato early blight, tomato gray mold, cucumber wilt, rice Pyricularia or rice sheath blight.
Embodiment 1
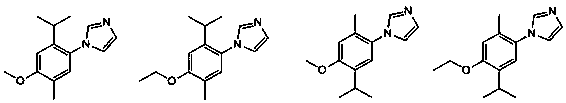
[0039] Embodiment 1: phenylimidazole compound (C 15 h 20 N 2 O) Synthesis.
[0040] The concrete steps of the synthetic method of this phenylimidazole derivative are as follows:
[0041] (1) Synthesis of intermediate 4-isopropyl-3-ethoxytoluene
[0042] In a 10 mL round-bottomed flask, 10 mmol of thymol was dissolved in 2 mL of DMF. After the dissolution was complete, 12 mmol of potassium carbonate and 12 mmol of ethyl bromide were added, and the reaction was stirred at room temperature for 15 h. The reaction was monitored by TLC. After the reaction was completed, distilled water was added, extracted with ethyl acetate, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure. The obtained crude product was separated and purified by column chromatography (petroleum ether: ethyl acetate = 10:1) to obtain the intermediate 4-isopropyl-3-ethoxytoluene.
[0043] (2) Synthesis of intermediate 2-methyl-5-isopropyl-4-ethoxybromobenzene
[0044]10 m...
Embodiment 2
[0056] Embodiment 2: phenylimidazole compound (C 14 h 18 N 2 O) Synthesis.
[0057] The concrete steps of the synthetic method of this phenylimidazole derivative are as follows:
[0058] (1) Synthesis of intermediate 4-isopropyl-2-methoxytoluene
[0059] In a 10 mL round-bottomed flask, 10 mmol of carvacrol was dissolved in 2 mL of DMF. After the dissolution was complete, 12 mmol of potassium carbonate and 12 mmol of methyl iodide were added, and the reaction was stirred at room temperature for 24 h. The reaction was monitored by TLC. After the reaction was completed, distilled water was added, extracted with ethyl acetate, dried over anhydrous sodium sulfate, and evaporated to dryness under reduced pressure. The obtained crude product was separated and purified by column chromatography to obtain the intermediate 4-isopropyl-2-methoxytoluene.
[0060] (2) Synthesis of intermediate 5-methyl-2-isopropyl-4-methoxybromobenzene
[0061] 10 mmol of the intermediate 4-isoprop...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com