Acid protease Bs2688 mutant Y282L with improved thermostability and gene and application thereof
A technology of acid protease and thermal stability, applied in the field of agricultural biology, can solve the problems of low catalytic efficiency and low enzyme activity of acid protease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Example 1 Preparation of protease Bs2688 mutant
[0045] 1. Clone the protease encoding gene Bs2688
[0046] The fungus Bispora sp.MEY-1, which has been cultured in liquid for 3 days, is centrifuged at 12,000 rpm for 10 minutes, and the collected mycelium is added to a high-temperature sterilized mortar, and quickly ground to powder with liquid nitrogen, and then the ground bacteria are transferred Put it in a new 50mL centrifuge tube containing 15ml CTAB lysis solution, gently mix upside down, place it in a 65℃ water bath for 3h, and gently mix upside down every 20 minutes to fully lyse the bacteria. Centrifuge at 4°C and 12,000 rpm for 10 minutes, transfer the supernatant to a new centrifuge tube, add an equal volume of chloroform for extraction, and leave at room temperature for 5 minutes. Centrifuge at 4°C and 12,000 rpm for 10 minutes. Take the supernatant and add an equal volume of phenol / chloroform for extraction, and leave it at room temperature for 5 min. Centrif...
Embodiment 2
[0066] Example 2. Verify the enzymatic performance of the recombinant protease
[0067] The activity analysis of the protease of the present invention is carried out by using the Folin phenol reagent color method. The specific method is as follows: Under the condition of pH3.0 and 55℃, a 1mL reaction system includes 500μL of appropriate diluted enzyme solution, 500μL of substrate, and react for 10min. Add 1mL of trichloroacetic acid (0.4mol / L) to terminate the reaction; The system was centrifuged at 12000 rpm for 3 minutes, 500 μL of supernatant was sucked into 2.5 mL of sodium carbonate (0.4 mol / L), and 500 μL of Phenol reagent was added. The color was developed at 40°C for 20 minutes and the OD value was measured at 680 nm after cooling. Definition of protease activity unit: Under certain conditions, the amount of enzyme required to decompose the substrate casein to produce 1 μmol tyrosine per minute is 1 activity unit (U).
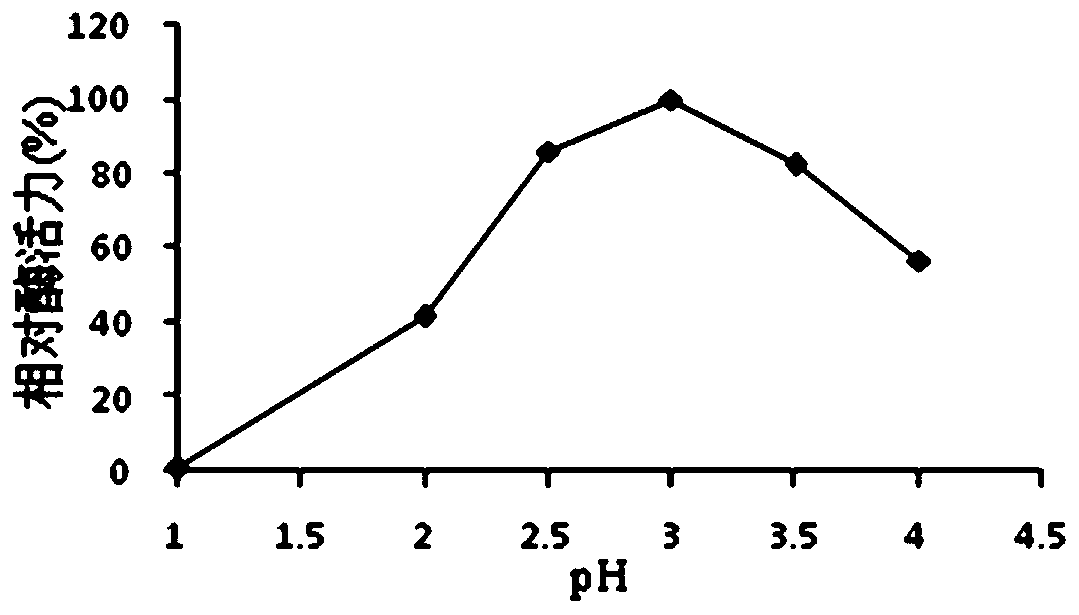
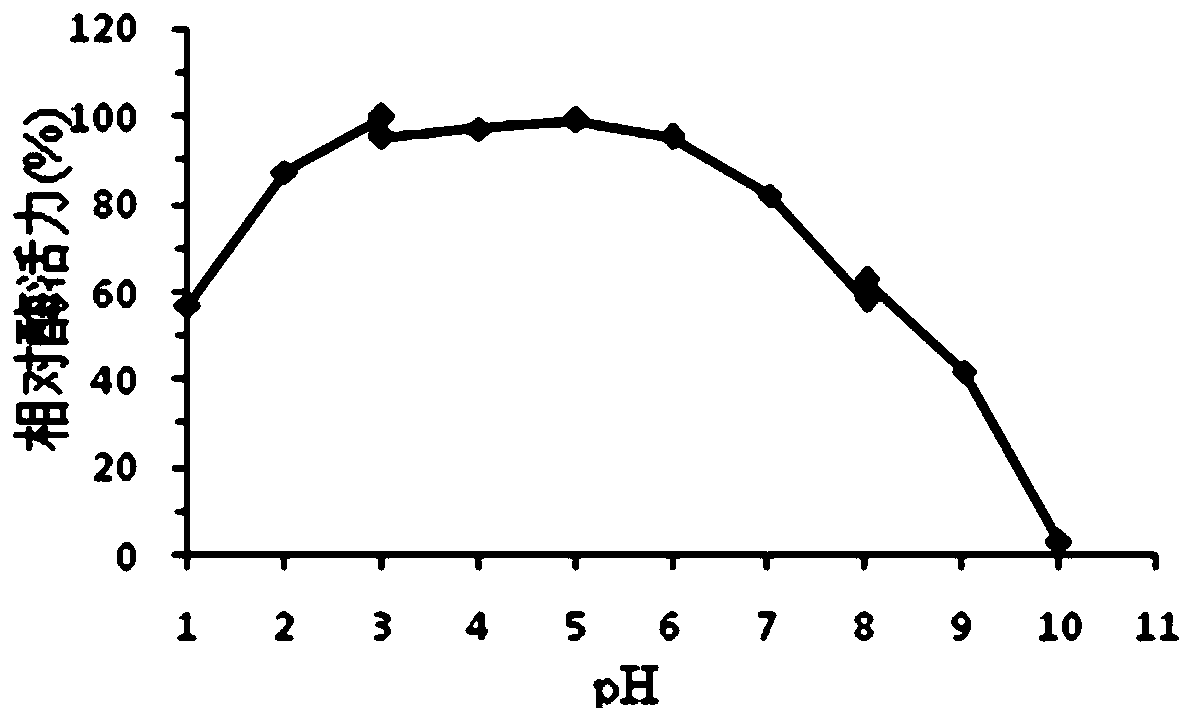
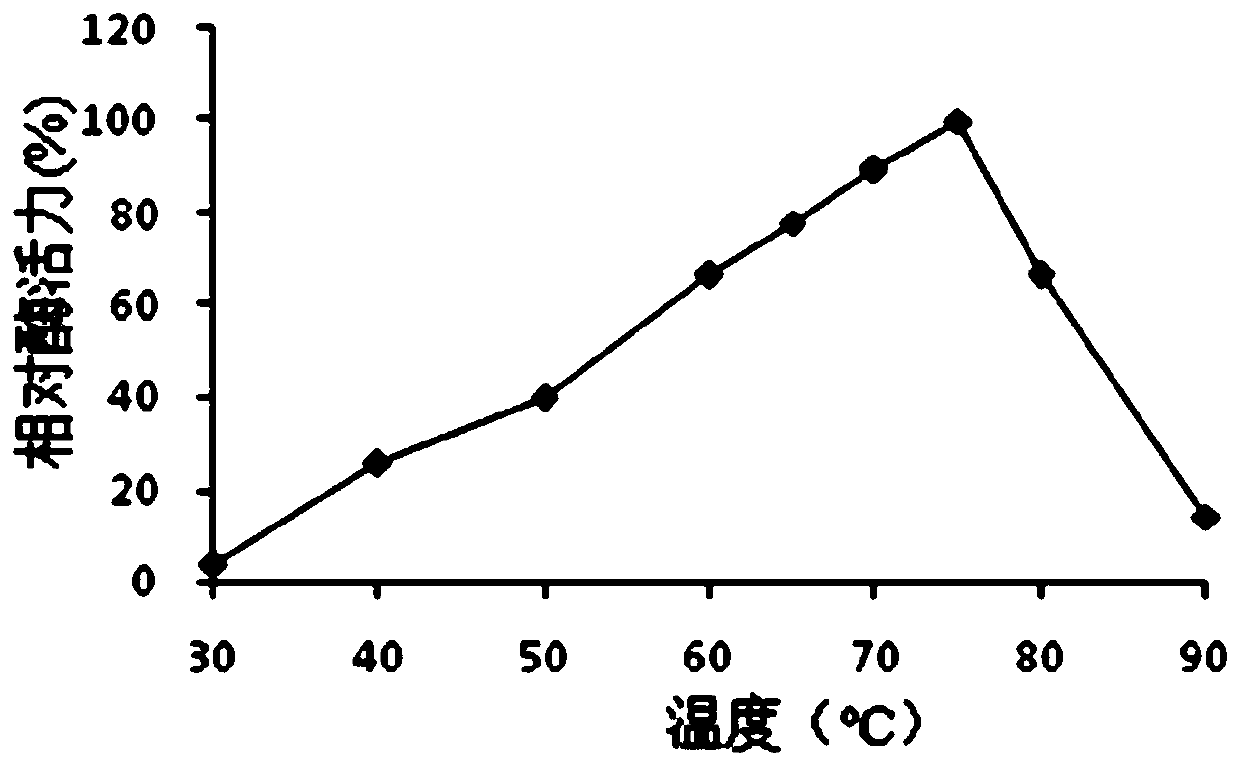
[0068] 1. Optimal pH and pH stability of protease Bs2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com