Method for recovering sodium carbonate from sodium tungstate leaching solution
A technology from sodium tungstate and sodium carbonate, applied in the direction of alkali metal carbonate, chemical instruments and methods, tungsten compounds, etc., can solve the problems of high production cost and environmental pollution, improve recovery rate, reduce cost, realize The effect of industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
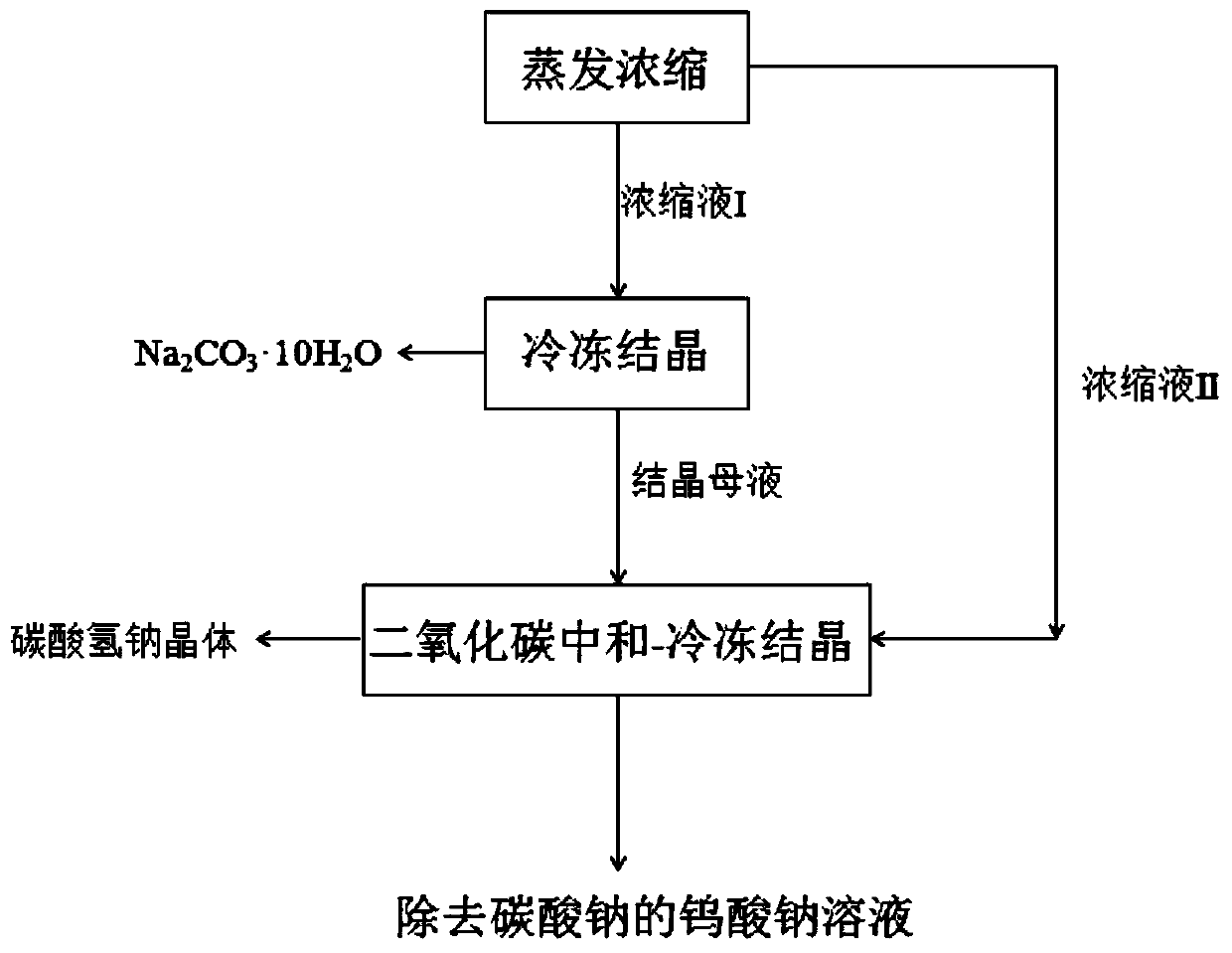
[0032] A kind of method that present embodiment reclaims sodium carbonate from sodium tungstate leaching solution, comprises the steps:
[0033] Step 1, evaporation and concentration:
[0034] Take 2000ml containing WO 3 40g / L, Na 2 CO 3 / WO 3 (mass concentration ratio) = 1.0 sodium tungstate leaching solution, concentrated by evaporation to a solution volume of 500ml, which contains WO 3 160g / L, containing Na 2 CO 3 160g / L, obtain concentrate I;
[0035] Step 2, freeze crystallization:
[0036] Cool the concentrated liquid I. When the temperature drops to 25°C, add 62g of crushed ice while stirring. After the crushed ice is completely dissolved within 3 minutes, the temperature drops to 16°C. After -1°C, continue to keep warm for 2.0 hours, and obtain sodium carbonate crystals and crystallization mother liquor after filtration, wherein the sodium carbonate crystals contain Na 2 CO 3 33%, containing WO 3 1.2%, Na in crystallization mother liquor 2 CO 3 / WO 3 (...
Embodiment 2
[0040] A kind of method that present embodiment reclaims sodium carbonate from sodium tungstate leaching solution, comprises the steps:
[0041] Step 1, evaporation and concentration:
[0042] Take 2000ml containing WO 3 110g / L, Na 2 CO 3 / WO 3 (mass concentration ratio) = 0.7 sodium tungstate leaching solution, concentrated by evaporation to a solution volume of 1000ml, which contains WO 3 220g / L, containing Na 2 CO 3 154g / L, obtain concentrate I;
[0043] Step 2, freeze crystallization:
[0044] Cool the concentrated solution I, when the temperature drops to 10°C, adjust the rotating speed to 6 rpm, continue to freeze and cool down to 0°C, add 30g of sodium carbonate crystals as a seed crystal, and continue to insulate for 1.5 hours, and obtain Sodium carbonate crystals and crystallization mother liquor, wherein sodium carbonate crystals contain Na 2 CO 3 32%, containing WO 3 1.9%, Na in crystallization mother liquor 2 CO 3 / WO 3 (mass concentration ratio) dro...
Embodiment 3
[0048] A kind of method that present embodiment reclaims sodium carbonate from sodium tungstate leaching solution, comprises the steps:
[0049] Step 1, evaporation and concentration:
[0050] Take 2000ml containing WO 3 96g / L, Na 2 CO 3 / WO 3 (mass concentration ratio) = 1.5 sodium tungstate leaching solution, concentrated by evaporation to a solution volume of 1400ml, which contains WO 3 137g / L, containing Na 2 CO 3 206g / L, obtain concentrate I;
[0051] Step 2, freeze crystallization:
[0052] Cool the concentrated liquid I, when the temperature drops to 15°C, adjust the rotating speed to 15 rpm, continue to freeze and cool down to 5°C, and continue to keep warm for 1.0 hour, after filtration, sodium carbonate crystals and crystallization mother liquor are obtained, of which sodium carbonate Crystal contains Na 2 CO 3 34%, containing WO 3 0.9%, Na in crystallization mother liquor 2 CO 3 / WO 3 (mass concentration ratio) drops to 0.36, and the crystallization...
PUM
| Property | Measurement | Unit |
|---|---|---|
| stone rate | aaaaa | aaaaa |
| stone rate | aaaaa | aaaaa |
| stone rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com