Lomefloxacin hydrochloride eye drops and preparation technology thereof
A technology of lomefloxacin hydrochloride and eye drops, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, organic active ingredients, etc. Poor effect, unable to significantly reduce intraocular pressure and other problems, to achieve excellent physical and chemical properties, reduce irritation, ensure the effect of sterile environment and safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
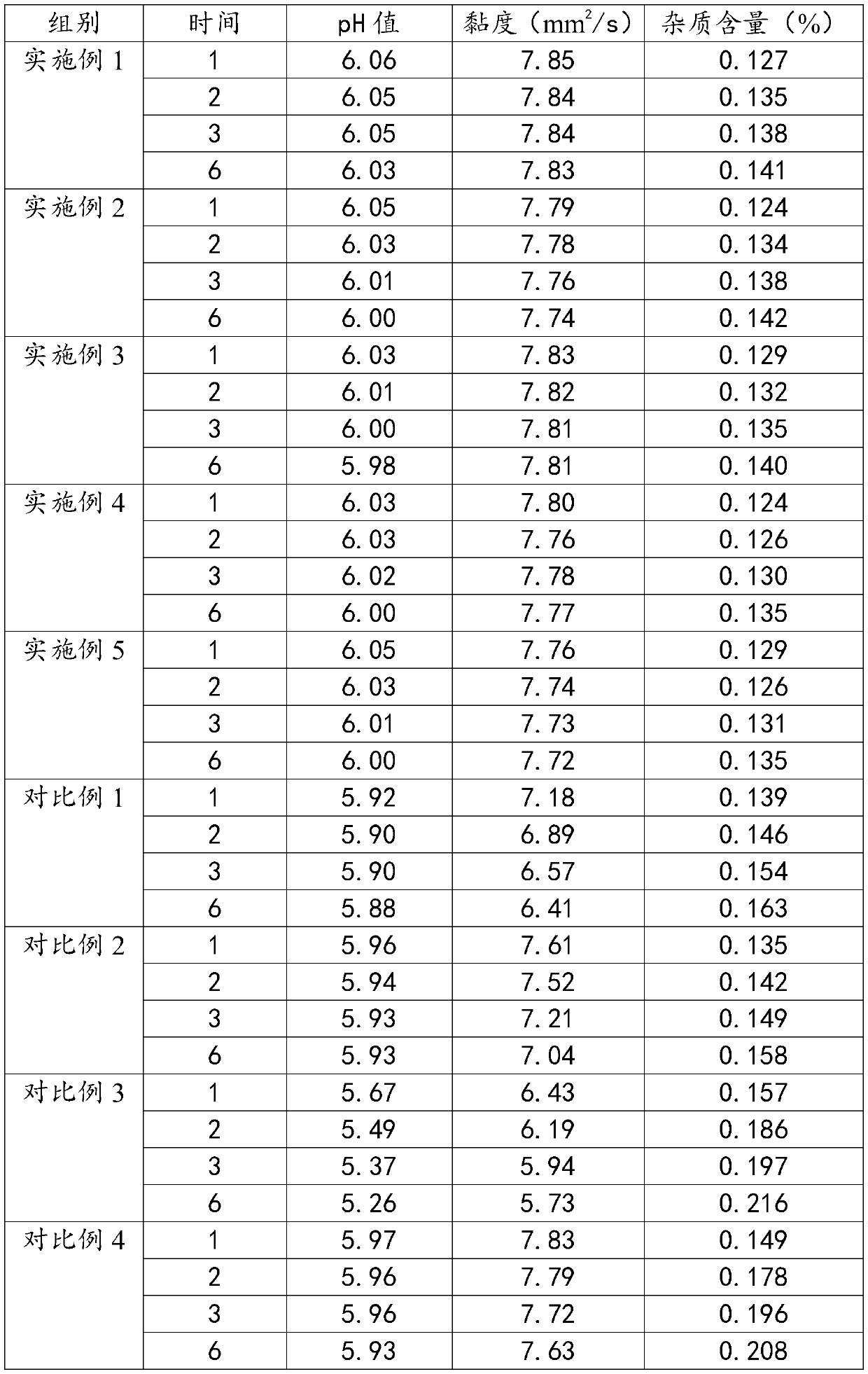
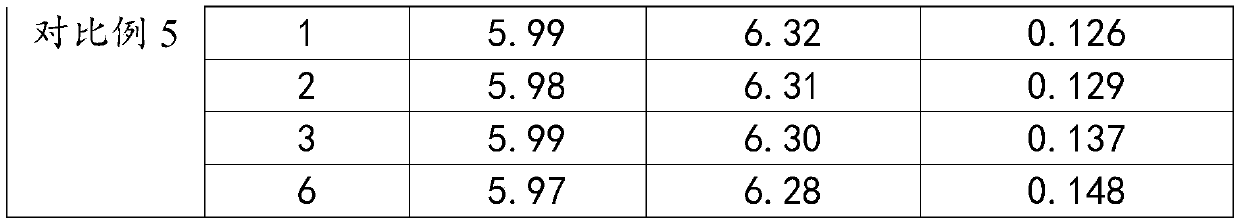
Embodiment 1
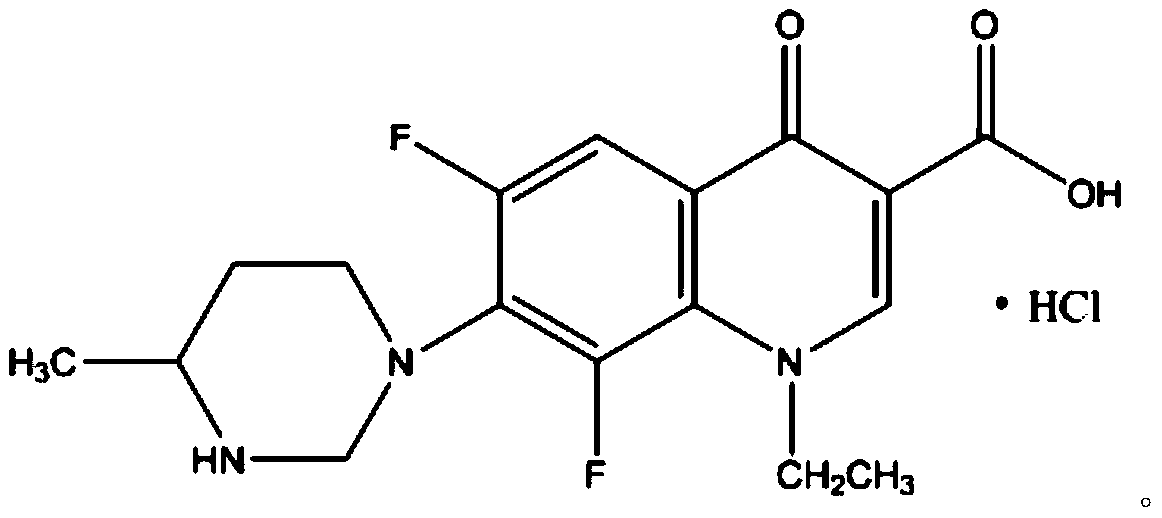
[0032] A kind of lomefloxacin hydrochloride eye drops of the present embodiment is prepared from the following substances in mass percentage: 0.36% of lomefloxacin hydrochloride, 4.2% of solubilizing additives, 0.07% of propyl p-hydroxybenzoate, 0.05% of sodium alginate %, glycerin 0.16%, buffer 0.016%, mannitol 0.046%, biodegradation aid 0.25%, and the balance is water for injection.
[0033] The preparation method of this eye drop comprises the following steps:
[0034] S1. Disperse sodium alginate and solubilizing additives in an appropriate amount of water for injection preheated to 52°C to make the system swell into a transparent solution;
[0035] S2. Disperse lomefloxacin hydrochloride in an appropriate amount of water for injection, mix it with the transparent solution preheated to 38°C and stir evenly, then add glycerin, mannitol, biodegradation additives and appropriate amount of water for injection, stir and mix, add Buffer to adjust pH and add remaining water for ...
Embodiment 2
[0043] A kind of lomefloxacin hydrochloride eye drops of the present embodiment is prepared from the following substances in mass percentage: 0.42% of lomefloxacin hydrochloride, 4.5% of solubilizing additives, 0.09% of propyl p-hydroxybenzoate, 0.06% of sodium alginate %, glycerin 0.17%, buffer 0.017%, mannitol 0.058%, biodegradation aid 0.28%, and the balance is water for injection.
[0044] The preparation method of this eye drop comprises the following steps:
[0045] S1. Disperse sodium alginate and solubilizing additives in an appropriate amount of water for injection preheated to 53°C to make the system swell into a transparent solution;
[0046] S2. Disperse lomefloxacin hydrochloride in an appropriate amount of water for injection, mix it with the transparent solution preheated to 43°C and stir evenly, then add glycerin, mannitol, biodegradation aids and appropriate amount of water for injection, stir and mix, add Buffer to adjust pH and add remaining water for injec...
Embodiment 3
[0054] A kind of lomefloxacin hydrochloride eye drops of the present embodiment is prepared from the following substances in mass percentage: 0.45% of lomefloxacin hydrochloride, 5.3% of solubilizing additive, 0.10% of propyl p-hydroxybenzoate, 0.07% of sodium alginate %, glycerin 0.18%, buffer 0.023%, mannitol 0.055%, biodegradation aid 0.28%, and the balance is water for injection.
[0055] The preparation method of this eye drop comprises the following steps:
[0056] S1. Disperse sodium alginate and solubilizing additives in an appropriate amount of water for injection preheated to 49°C to make the system swell into a transparent solution;
[0057] S2. Disperse lomefloxacin hydrochloride in an appropriate amount of water for injection, mix it with the transparent solution preheated to 42°C and stir evenly, then add glycerin, mannitol, biodegradation additives and appropriate amount of water for injection, stir and mix, add Buffer to adjust pH and add remaining water for i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com