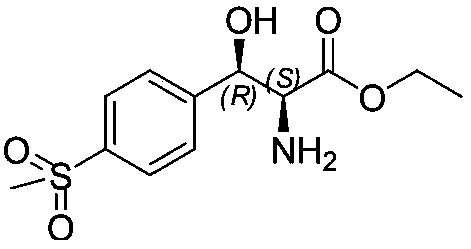
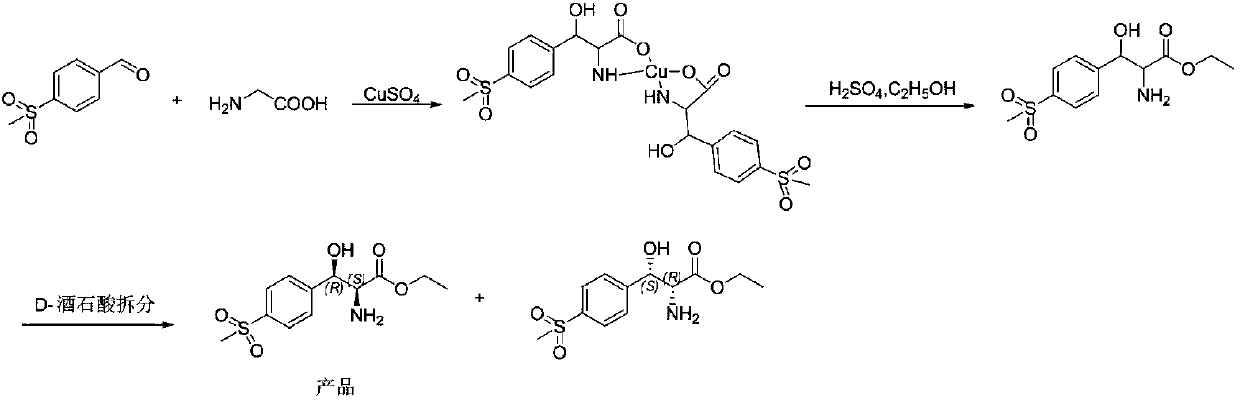
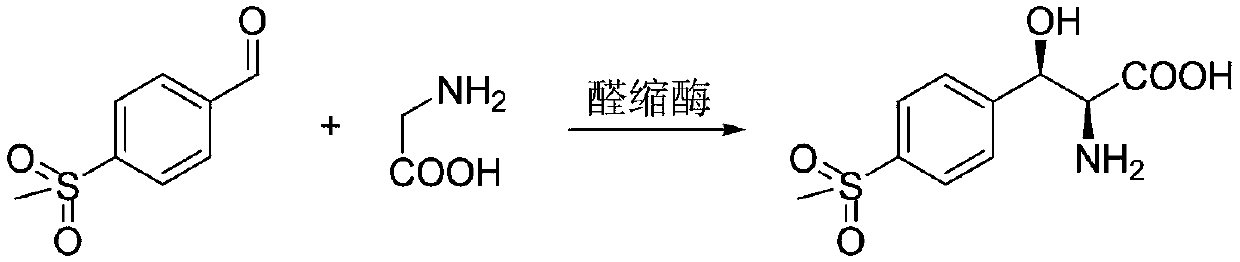
Method for preparing chiral (2S,3R)-p-methyl sulfone phenyl ethyl serinate
A technology of thiamphenyl phenylserine ethyl ester and p-methylsulfone group, which is applied in the field of preparation of chiral-p-thiamphenyl phenylserine ethyl ester, which can solve the problems of large environmental hazards, cumbersome resolution steps, poor stereoselectivity, etc. problems, to achieve the effect of cost reduction, simple operation and mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] In a 250mL reaction flask, add 2g p-thymphenylbenzaldehyde, 1.55gL-threonine, 0.1g magnesium chloride, 4mg pyridoxal phosphate, 100mL100mM phosphate buffer solution (pH7.0) and heat to 30°C with magnetic stirring until uniform, add 10mg transaldolase enzyme powder (purchased from Suzhou Pivot Biotechnology Co., Ltd., product number is YH1058, here only provides one of the products of the type to illustrate the effect of the present invention), start stirring reaction, and sample HPLC detection after 20 hours The conversion rate is above 95%. After the reaction was completed, the temperature in the reaction system was slowly raised to 70-80° C. and stirred for 0.5 hours, filtered (diatomaceous earth filter), and the filtrate was concentrated to 10 ml. The resulting aqueous solution was adjusted to pH 11 with 40% NaOH, and 0.6 equivalent of CaCl was added 2 , stirred at room temperature for 2-3 hours, at which time a large amount of white solid precipitated. The tempera...
Embodiment 2
[0033] Take 2 g of the above white solid crude product, add 20 ml of ethanol, and cool the system down to 0°C. Slowly add 1.2 equivalents of sulfuric acid to the reaction system dropwise. After the dropwise addition, the temperature of the system was raised to reflux, and the temperature was lowered to room temperature after reflux for 8 hours. Filter to remove calcium sulfate. The filtrate was concentrated to 5ml and neutralized with aqueous ammonia to pH=8.5. The system was cooled to 0°C and stirred for 1 hour, and a large amount of white solid precipitated out. Filtered, washed with a small amount of ice water, sucked dry, and dried under vacuum at 60°C to obtain 1.71 g of (2S,3R)-p-methylsulfonylphenylserine ethyl ester with a yield of 83%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com