A-D-A type benzothiadiazole small molecule, preparation method and application thereof
A small benzothiadiazole, A-D-A technology, applied in the field of A-D-A type benzothiadiazole small molecules and their preparation, can solve the problems of high manufacturing cost, poor durability, disordered structure, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] The preparation method of the A-D-A type benzothiadiazole small molecule provided in the embodiment of the present invention includes:
[0047] S101: Preparation of reaction raw materials and reaction conditions; the reaction raw materials are: benzothiadiazoles with different numbers of fluorine (structural formulas are shown in formulas IV, V, and VI), electron-donating monomers, and catalysts;
[0048] S102: In an organic solvent system, under the protection of an inert gas, conduct a reaction in the dark;
[0049] S103: column chromatography purification;
[0050] S104: preparing the target product.
[0051] As a preferred embodiment of the present invention, step S101 specifically includes: the preparation method of the A-D-A type benzothiadiazole small molecule is specifically: from benzothiadiazoles with different numbers of fluorine (structural formulas such as formulas IV, V, Ⅵ) respectively react with electron-donating monomers and catalysts in an organic so...
Embodiment 1
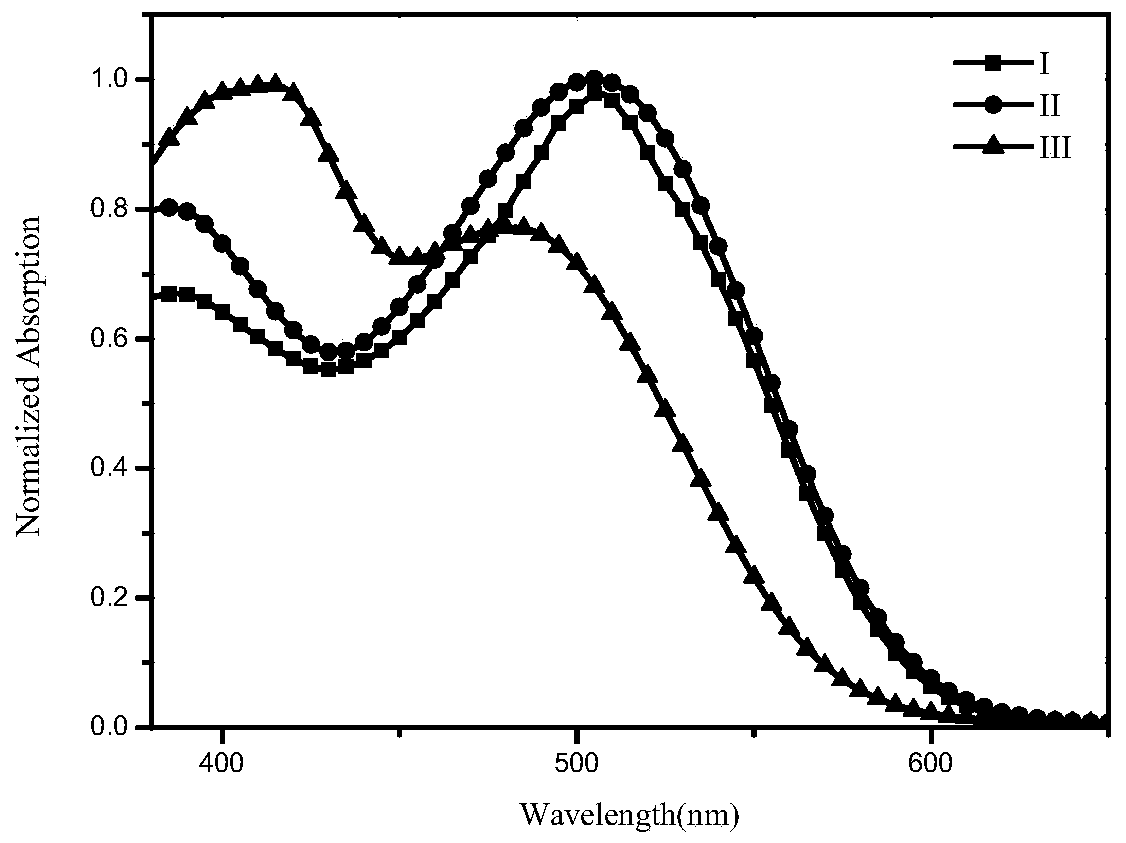
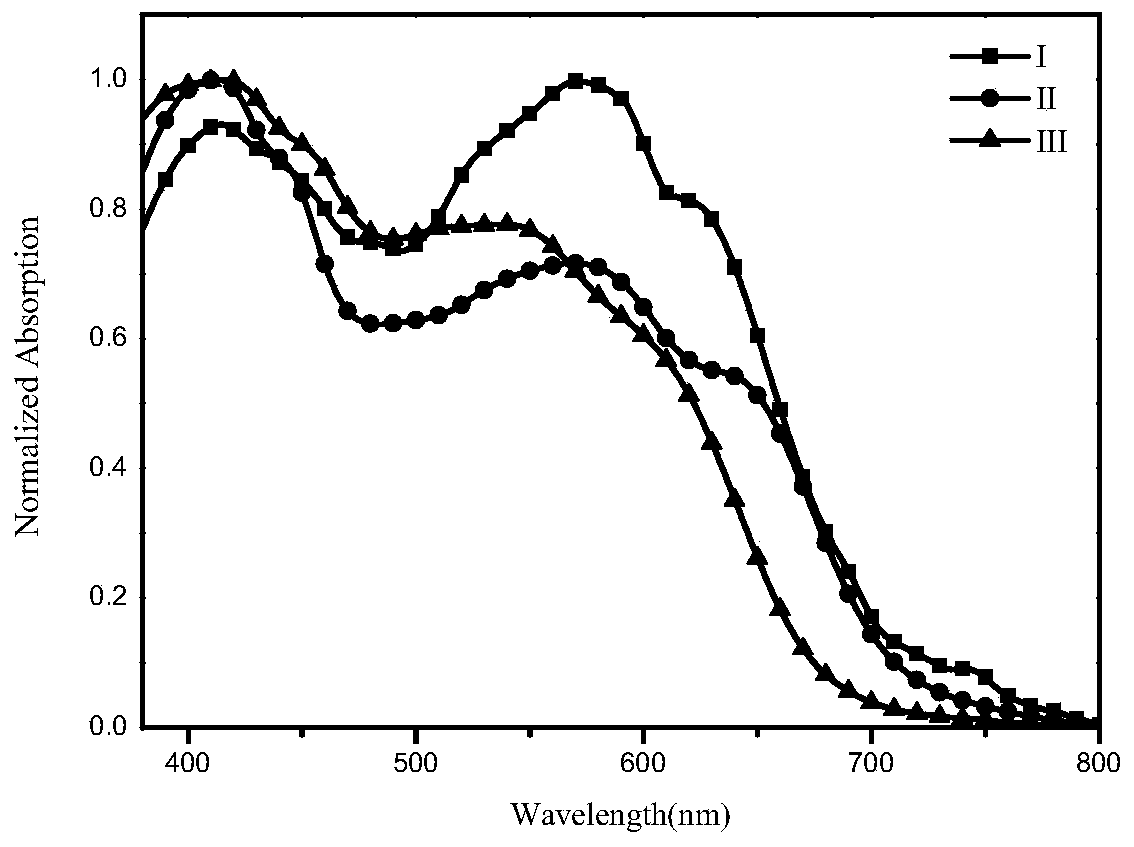
[0071] The present invention provides a small molecule of A-D-A benzothiadiazole, the structural formula of which is formula I, as follows:
[0072]
[0073] The A-D-A type benzothiadiazole small molecular formula I provided in this example is prepared by the following method:
[0074]1 mol of the compound shown in formula IV and 0.5 mol (4,8-bis(octyloxy)benzo[1,2-B:4,5-B']dithiophene-2,6-diyl)di (Tributyltin) is placed in a toluene solvent, using tris(2-methylphenyl)phosphine as a catalyst, under the protection of an inert gas, the temperature is controlled at 110°C, the reaction is protected from light for 72 hours, and the target product formula I is obtained by column chromatography purification ; 1H NMR (CDCl3, 400MHz, δ / ppm): 8.03(d, 4H), 7.81(s, 4H), 7.63(s, 2H), 7.08(s, 2H), 4.41(t, 4H), 3.02( t, 4H), 2.75(, 4H), 1.75-2.35(m, 10H), 1.4-1.75(m, 34H), 0.75-1.75(m, 30H); 13CNMR(CDCl3, 100MHz, δ / ppm): 154.44 . The synthetic route of described formula I is as follows...
Embodiment 2
[0082] The present invention provides a small molecule of A-D-A benzothiadiazole, the structural formula of which is formula II, as shown below:
[0083]
[0084] The A-D-A type benzothiadiazole small molecular formula II provided in this example is prepared by the following method: 1 mol of the compound shown in formula V and 0.5 mol (4,8-bis(octyloxy)benzo[1,2 -B: 4,5-B']dithiophene-2,6-diyl)bis(tributyltin) is placed in toluene solvent, copper oxide is used as catalyst, under the protection of inert gas, the temperature is controlled at 110°C, avoiding Light reaction for 72h, column chromatography purification to obtain the target product formula II; 1HNMR (CDCl3, 400MHz, δ / ppm): 8.23 (s, 4H), 7.85 (s, H), 7.75 (s, 3H), 7.53 (s, 3H), 4.26(t,4H), 2.91(t,4H), 2.63(t,4H), 1.51-2.25(m,22H), 0.75-1.51(m,52H); 13CNMR(CDCl3,100MHz,δ / ppm): 156.43, 146.86, 145.27, 141.11, 138.76, 137.72, 134.02, 132.25, 129.11, 127.88, 123.56, 117.27, 77.15, 35.98, 34.65, 32.41, 28.66, 24.131...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com