Neuropeptide-expressing vectors and methods for treatment of epilepsy
A technology for epilepsy and delivery vectors, which can be used in neurological diseases, chemical instruments and methods, biochemical equipment and methods, etc., and can solve problems such as unimproved seizure rate.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0244] 1.1 AAV construct
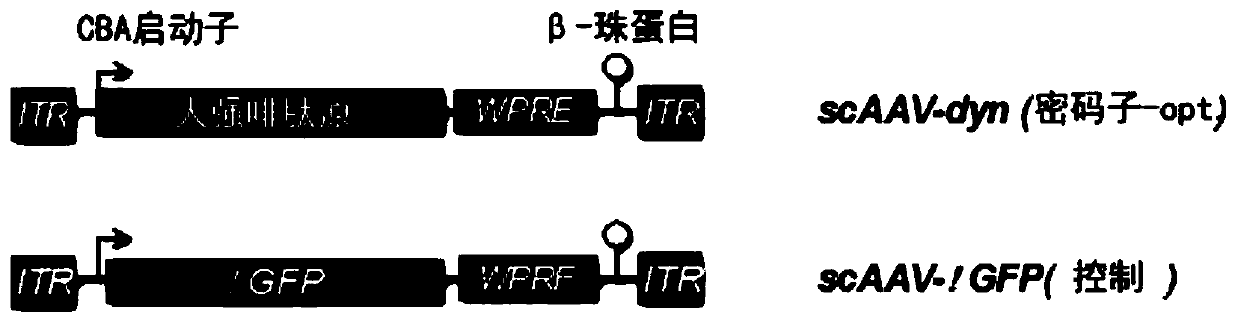
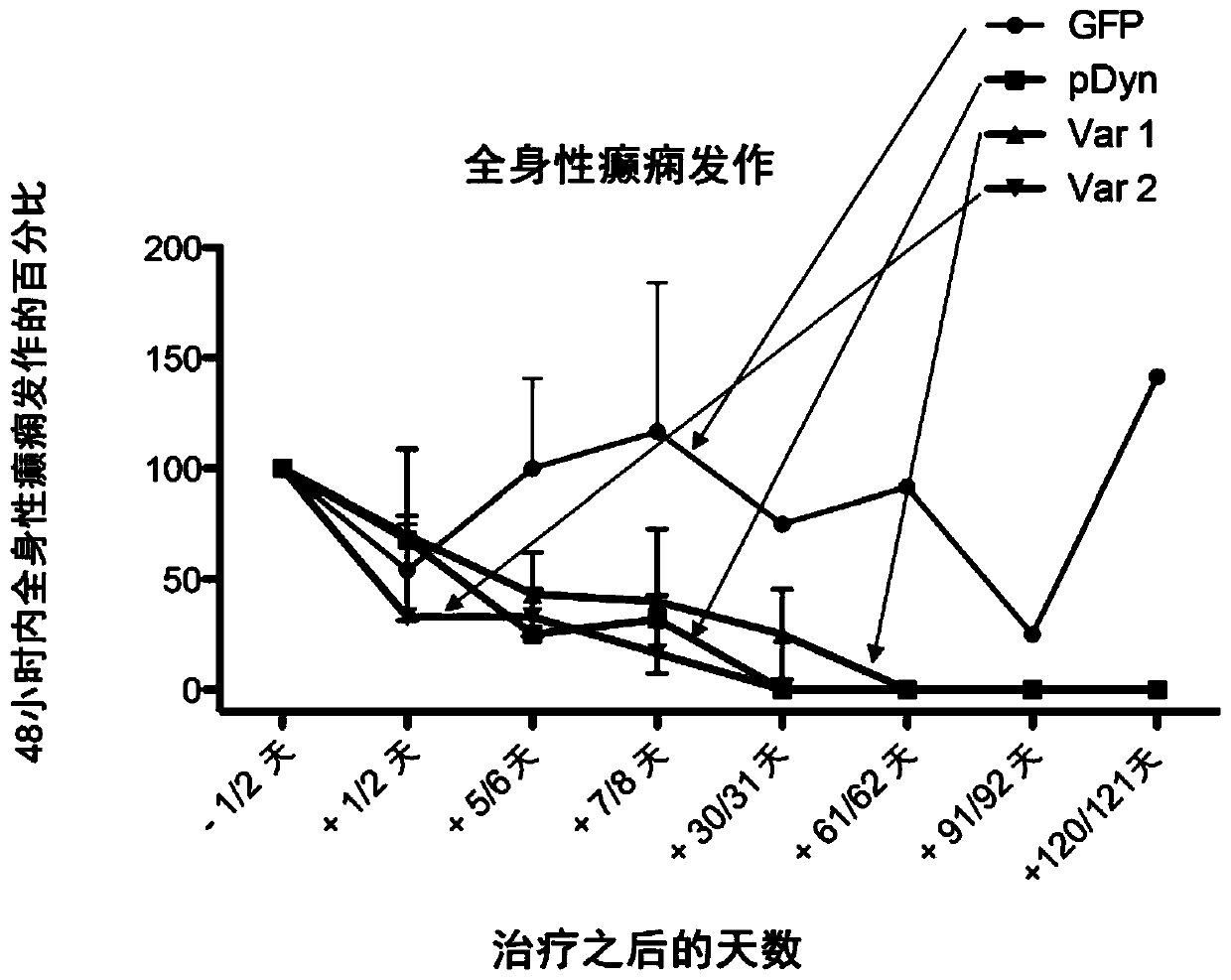
[0245] The plasmid with the AAV2-ITR flanking the vector backbone contains the human CMV-IE gene enhancer, followed by the truncated form of the chicken beta actin promoter. These drive full-length codon-optimized human ppdyn sequences (AA SEQ ID No. 1) or variants (AA SEQ ID No. 2; SEQ ID No. 3) or truncated variants (□GFP) Or expression of the full-length form of EGFP. Gene expression is enhanced by the woodchuck hepatitis virus post-translational enhancer element (WPRE), followed by poly A derived from bovine growth hormone + Signal sequence. AAV2ITR is in its wild-type configuration to produce an AAV vector with ssDNA genome, or one ITR is truncated to produce a self-complementary AAV vector with dsDNA genome ( figure 1 ).
[0246] 1.2 AAV vector preparation
[0247] HEK 293 cells were seeded in DMEM containing 5% FCS at 25% to 33% confluence. After 24 hours, the cells were transfected by calcium phosphate co-transfection. The production of AAV ve...
Embodiment 2
[0253] animal
[0254] C57BL / 6N wild-type and pDyn knockout (pDyn-KO) mice were investigated in this study. The pDyn-KO mice were backcrossed to the C57BL / 6N background for 10 generations (Loacker et al., 2007). In order to breed and maintain, the mice are raised in groups and have free access to food and water. The temperature was fixed at 23° C. and 60% humidity, with a 12-hour light-dark cycle (lighting from 7 am to 7 pm). All procedures involving animals were approved by the Austrian Animal Experimentation Ethics Board in accordance with the European Convention for the Protection of Vertebrates for Experimental and Other Scientific Purposes ETS Number: 123 and the Canadian Animal Protection Committee. We make every effort to reduce the number of animals used.
Embodiment 3
[0256] Kainic acid injection and electrode implantation
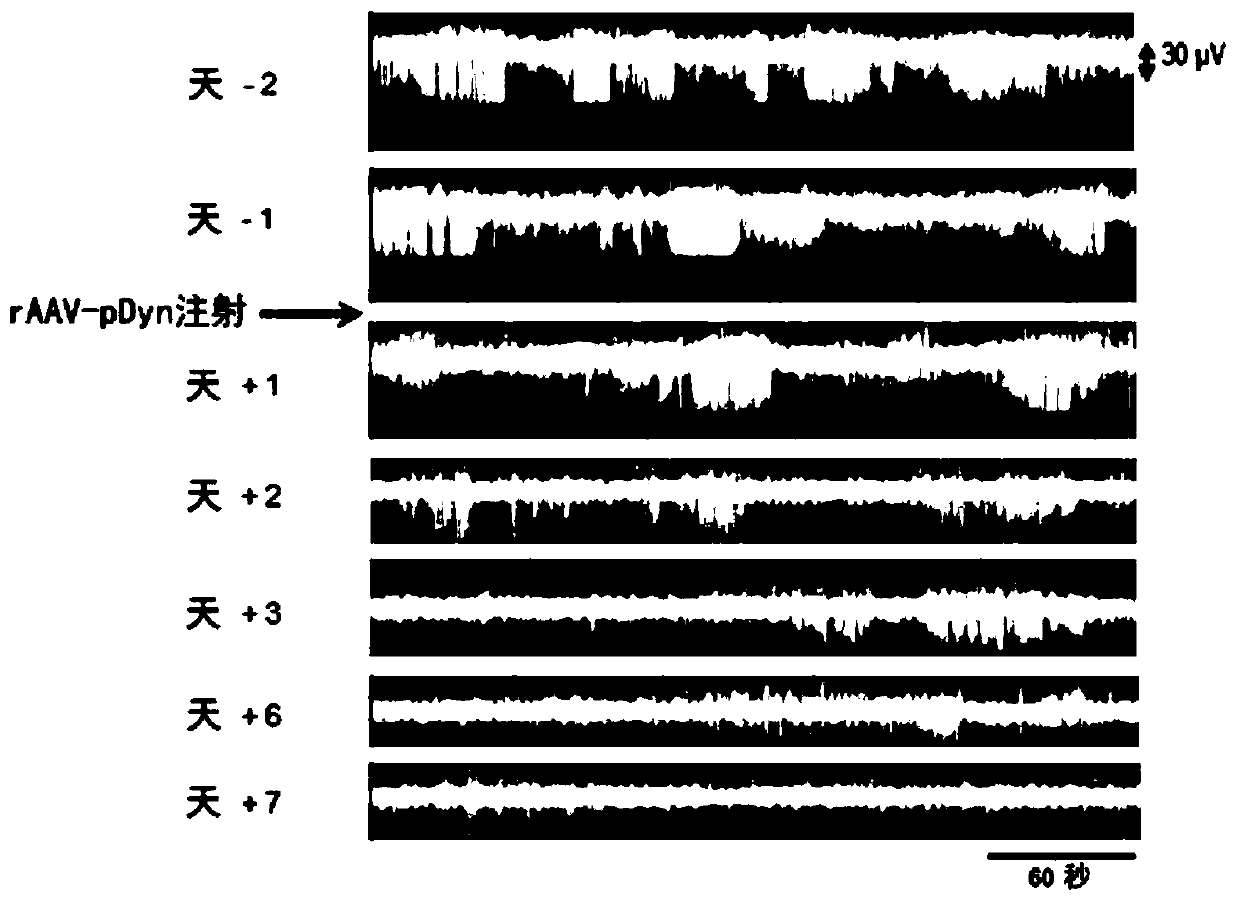
[0257] Male mice (12 to 14 weeks) were sedated with ketamine (160 mg / kg, i.p.; Graeub veterinary product, Switzerland), and then deeply anesthetized with sevoflurane through a precision vaporizer (Midmark, USA). As mentioned earlier, mice were injected with 50 nl20mM KA solution into the hippocampus (RC-1.80mm; ML+1.80mm; DV-1.60mm) (Loacker et al., 2007). Two electrodes (a cortical electrode and a depth electrode) were implanted immediately after KA administration. An epoxy resin-coated tungsten depth electrode (diameter 250 μm; FHC, USA) was placed in the hippocampus, targeting the CA1 area (RC-1.80mm; ML+1.80mm; DV-1.60mm). The surface electrode is a gold-plated screw (RC+1.70mm; ML+1.6mm, bregma as a reference point) placed in the skull on top of the motor cortex to monitor the summary of abnormal EEG activity. An additional surface electrode is placed on the cerebellum as ground and reference. The electrodes were f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com