A conjugated organic small molecule interface modification material, preparation method and organic solar cell composed thereof
An organic solar cell and interface modification technology, which is applied in the preparation of organic compounds, organic chemistry, carboxylate preparation, etc., to achieve the effects of reducing electron transport barriers, increasing extraction rates, and improving performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
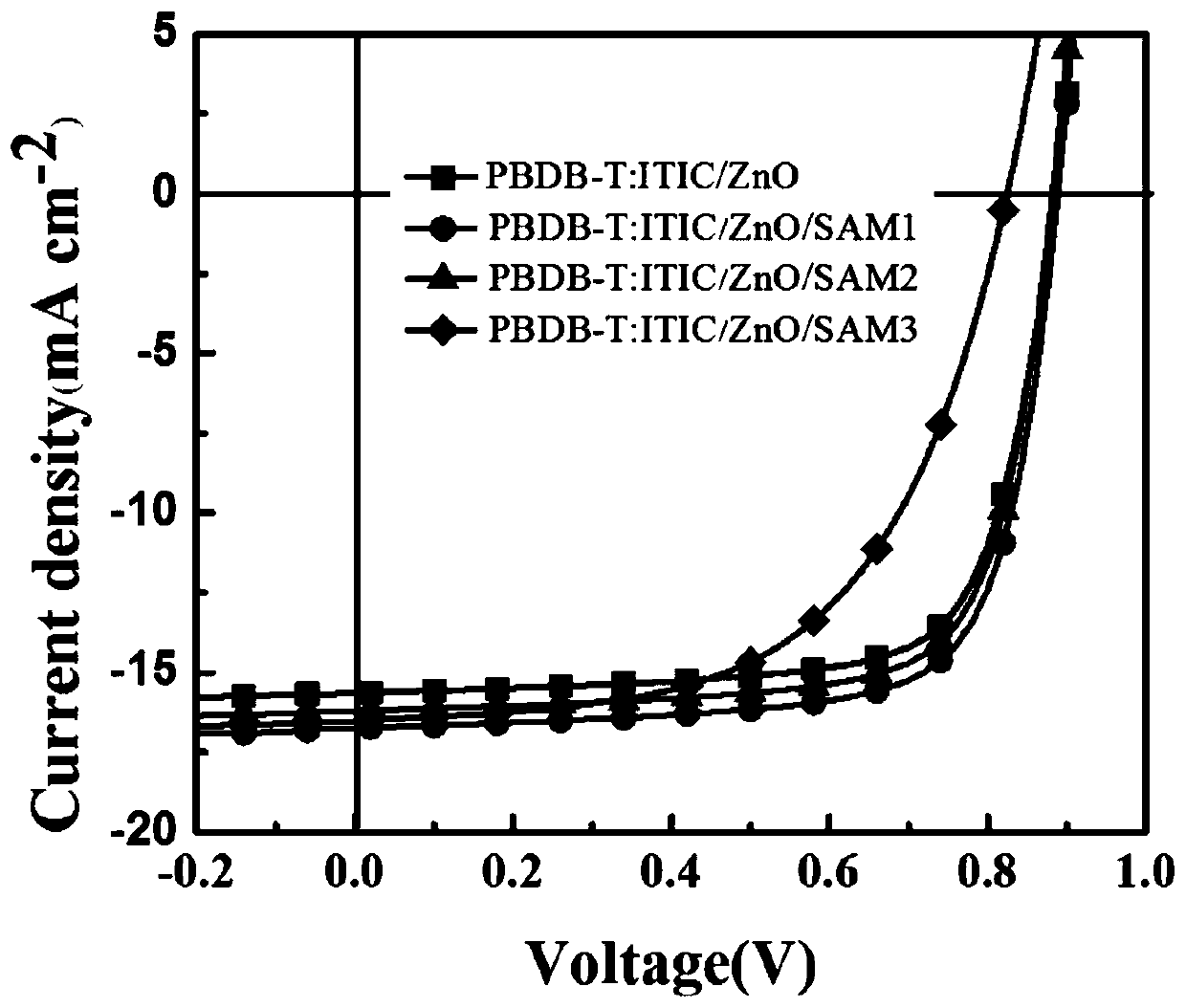
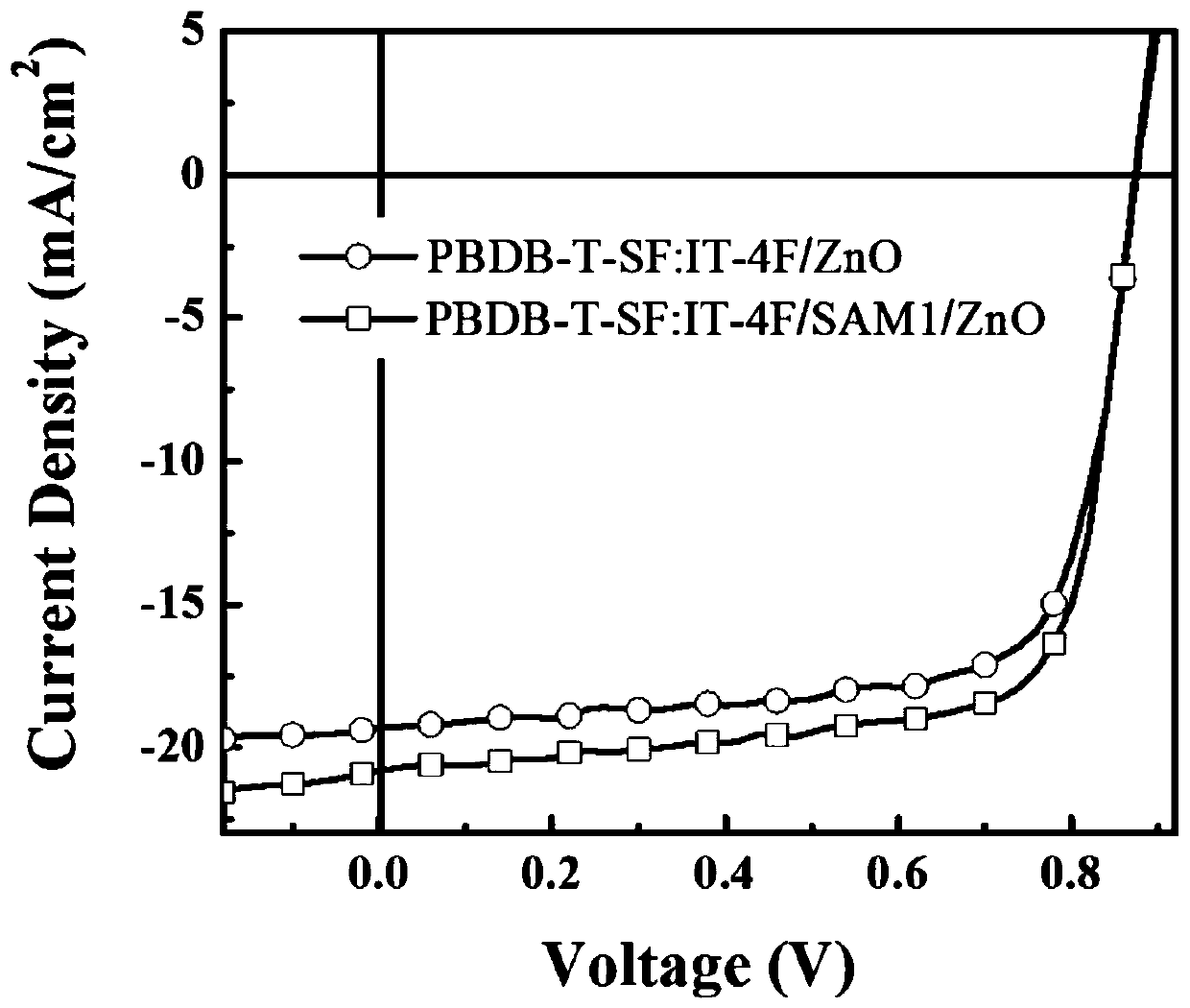
[0033] Add 1 molar equivalent of 1,3-indanedione, 1.2 molar equivalent of p-aldehyde benzoic acid and 0.1 molar equivalent of β-alanine into the dry reaction flask. Then according to the feeding amount, add 10ml of mixed solvent of ethanol and 1,2-dichloroethane for every 2mmol of 1,3-indanedione, wherein the volume ratio of ethanol to 1,2-dichloroethane is 1:1. Then react at 80°C under reflux for 8h. During the reaction, a yellow solid was precipitated, and after the reaction was completed, the precipitated solid was obtained by suction filtration. The solid was washed three times with a mixed solvent of ethanol and 1,2-dichloroethane to obtain SAM1. The NMR spectrum of SAM1 is as follows Figure 4 shown. 1 H NMR (DMSO-d6, 500MHz): δ13.28(s, 1H), 8.48(d, J=10Hz, 2H), 8.02(d, J=10Hz, 2H), 7.94-8.00(m, 4H), 7.86(s,1H).
Embodiment 2
[0035]Add 1 molar equivalent of rhodanine, 2 molar equivalents of p-aldehyde benzoic acid and 1 molar equivalent of β-alanine into the dry reaction bottle. Then according to the feeding amount, add 10ml of mixed solvent of ethanol and 1,2-dichloroethane for every 2mmol of rhodanine, wherein the volume ratio of ethanol and 1,2-dichloroethane is 1:1. Then react at 80°C under reflux for 8h. During the reaction, a yellow solid was precipitated, and after the reaction was completed, the precipitated solid was obtained by suction filtration. The solid was washed three times with a mixed solvent of ethanol and 1,2-dichloroethane to obtain SAM2. The NMR spectrum of SAM2 is as follows Figure 5 shown. 1 H NMR (DMSO-d 6 ,500MHz):δ8.05(d,J=10Hz,2H),7.85(s,1H),7.73(d,2H),4.06-4.10(m,4H),1.19(t,J=7.5Hz,3H ).
Embodiment 3
[0037] Add 1 molar equivalent of dicyanorhadanine, 1.6 molar equivalent of p-aldehyde benzoic acid and 0.6 molar equivalent of β-alanine in a dry reaction flask, wherein the volume ratio of ethanol to 1,2-dichloroethane is 1:1, wherein the volume ratio of ethanol to 1,2-dichloroethane is 1:1. Then according to the feeding amount, add 10ml of mixed solvent of ethanol and 1,2-dichloroethane for every 2mmol of dicyanorhadenine. Then react at 80°C under reflux for 8h. During the reaction, a yellow solid was precipitated, and after the reaction was completed, the precipitated solid was obtained by suction filtration. The solid was washed three times with a mixed solvent of ethanol and 1,2-dichloroethane to obtain SAM3. The NMR spectrum of SAM3 is as follows Image 6 shown. 1 H NMR (DMSO-d 6 , 500MHz): δ8.09-8.11 (m, 3H), 7.78 (d, J = 10Hz, 2H), 4.13-4.17 (m, 2H), 1.28-1.31 (t, J = 7.5Hz, 3H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com