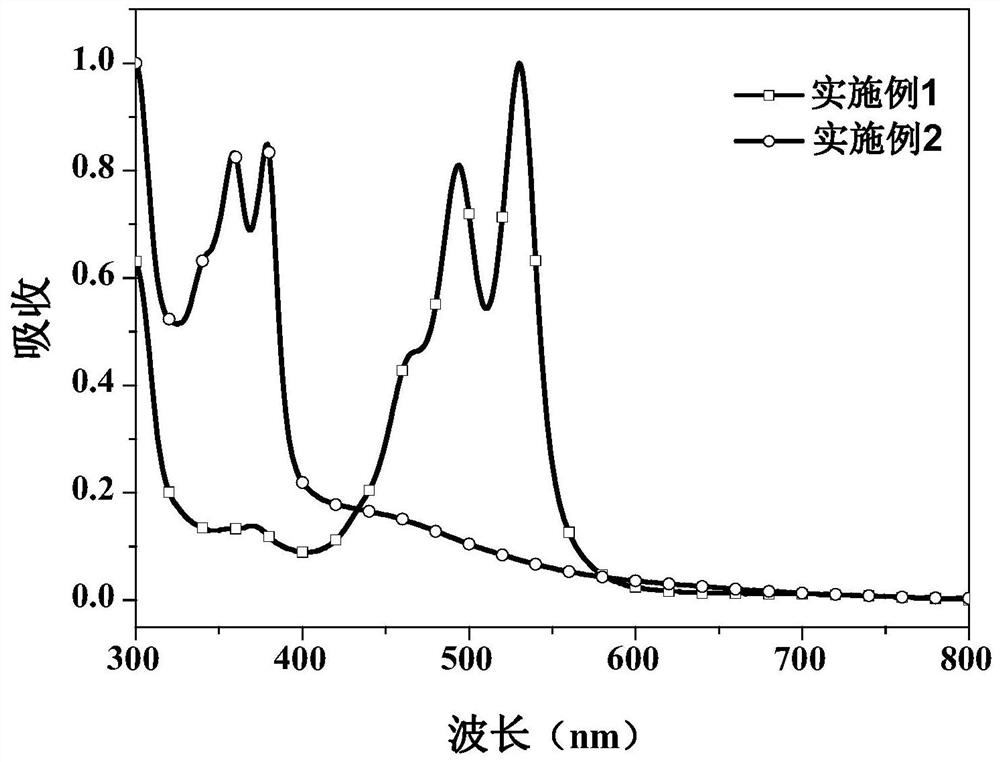
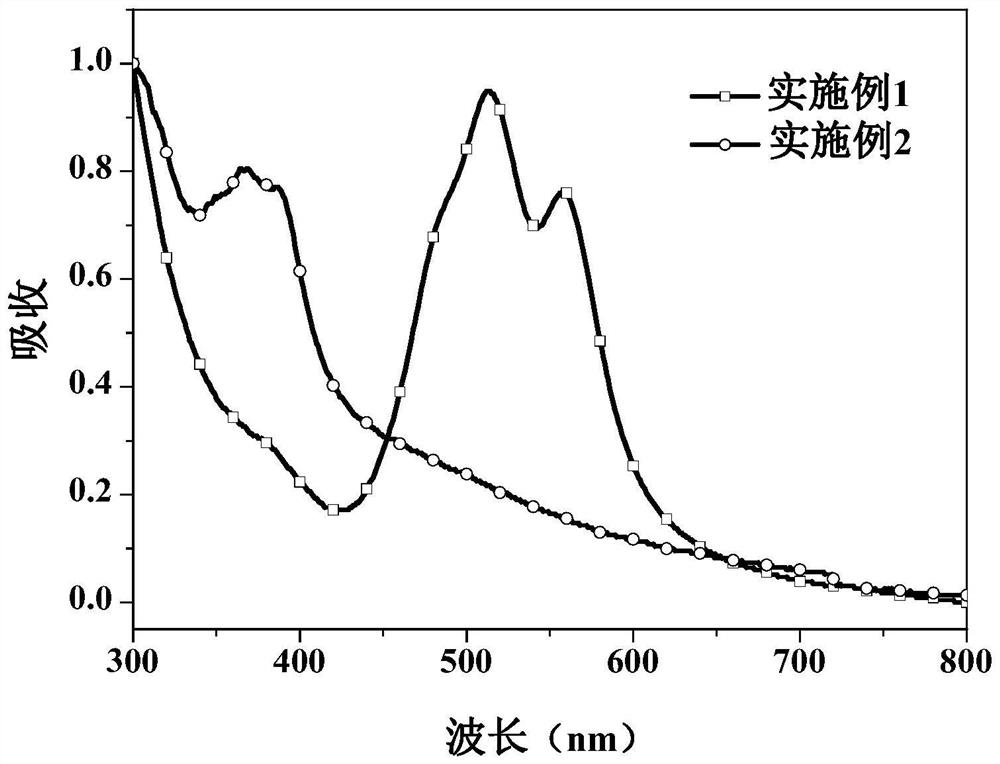
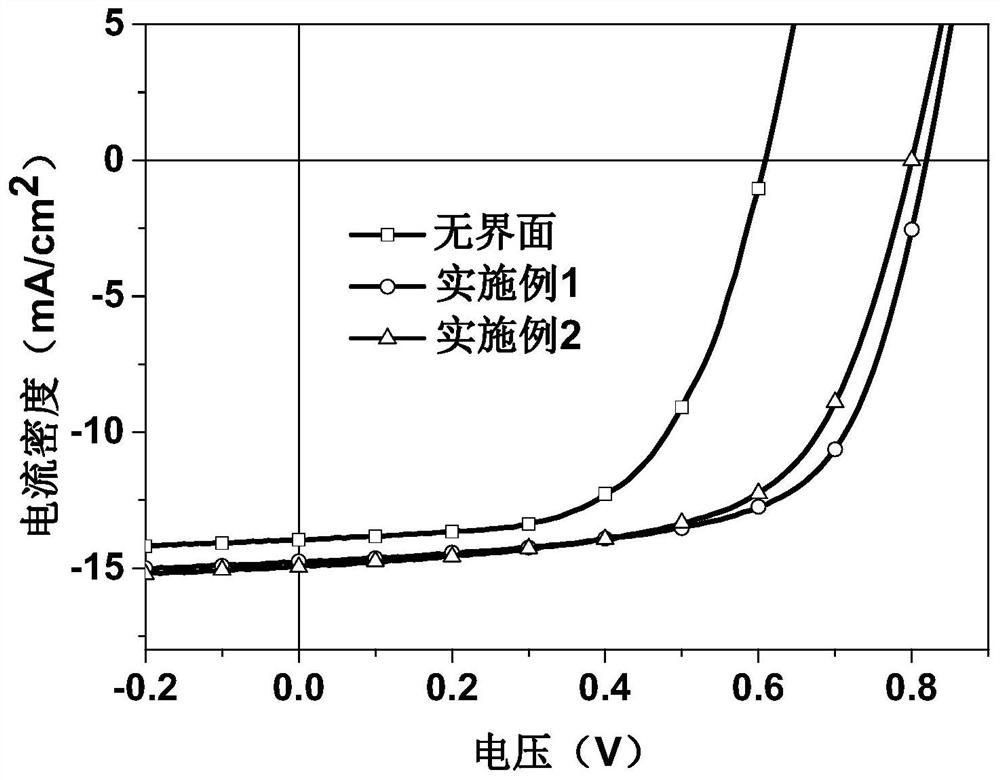
A kind of phenanthroline organic small molecule cathode interface material and preparation method thereof
A cathode interface, phenanthroline technology, applied in organic chemistry, semiconductor/solid-state device manufacturing, electric solid-state devices, etc., can solve the problems of high processing cost, crystalline silicon hardness, high melting point, application limitations, etc. function, good light transmittance, and the effect of improving photoelectric conversion efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] (1) Synthesis of N,N'-bis(5-phenanthroline)-3,4,9,10-perylimide
[0037]
[0038] In a 250mL two-necked round bottom flask, add 3,4,9,10-perylenetetracarboxylic dianhydride (1g, 2.55mmol), 5-amino-1,10-phenanthroline (2g, 10.24mmol), 80g imidazole and zinc acetate (93mg, 0.51mmol), blown with nitrogen for 10 minutes, heated to 160°C and stirred for 6 hours. After the reaction, a sufficient amount of water was added to precipitate, and the filter residue was obtained by filtration, washed with 2 wt % sodium hydroxide solution, and finally recrystallized with dimethyl sulfoxide to obtain a dark red solid. Mass(MALDI-TOF): Obs.747.07; Calcd.For C 48 h 22 N 6 o 4 ,746.74.
[0039] (2) Synthesis of N,N'-bis(5-phenanthroline)-3,4,9,10-perylimide dicarbobromide ion
[0040]
[0041] In an argon atmosphere, N,N'-bis(5-phenanthroline)-3,4,9,10-perylimide (207mg, 0.28mmol) was added to a 50ml two-necked round-bottomed flask, and then 15ml1, 2-Dibromoethane, protected...
Embodiment 2
[0047] (1) Synthesis of N,N'-bis(5-phenanthroline)-1,4,5,8-naphthalimide
[0048]
[0049] In a 250mL two-neck round bottom flask, add 1,4,5,8-naphthalene tetracarboxylic dianhydride (379mg, 1.41mmol), 5-amino-1,10-phenanthroline (827g, 4.24mmol) and 25g imidazole , Then add 50ml of pyridine and stir to dissolve, blow with nitrogen for 10 minutes, heat to 130°C and stir for 8h. After the reaction, the reaction solution was poured into 300ml of glacial acetic acid and stirred for 1 h, then enough water was added to continue stirring for 1 h, the solid was obtained by filtration, and then recrystallized with dimethyl sulfoxide to obtain a light gray solid. Mass(MALDI-TOF): Obs.623.23; Calcd.For C 38 h 18 N 6 o 4 ,622.60.
[0050] (2) Synthesis of N,N'-bis(5-phenanthroline)-1,4,5,8-naphthalimide dicarbobromide ion
[0051]
[0052] In an argon atmosphere, add N,N'-bis(5-phenanthroline)-1,4,5,8-naphthalimide (100mg, 0.16mmol) into a 50ml two-necked round-bottomed flask...
Embodiment 3
[0058] Synthesis of N,N'-bis(5-phenanthroline)-3,4,9,10-perylimide tricarbonyl bromide
[0059]
[0060] In an argon atmosphere, N,N'-bis(5-phenanthroline)-3,4,9,10-perylimide (207mg, 0.28mmol) was added to a 50ml two-necked round-bottomed flask, and then 15ml1, 3-Dibromopropane, protected from light, heated up to 150°C, and reacted for 6h. After the reaction was completed, a sufficient amount of tetrahydrofuran was added to precipitate a solid, and then recrystallized with dimethyl sulfoxide to obtain a dark red solid. Mass(MALDI-TOF): Obs.1151.43; Calcd.ForC 52 h 30 Br 4 N 6 o 4 ,1150.52.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com