Method for expressing and purifying recombinant human sex hormone haptoglobin N-terminal 51-218aa
A combination of globulin and expression method technology, applied in the field of expression and purification of recombinant human sex hormone binding globulin N-terminal 51-218aa, which can solve the problems of expensive kits and restrictions on the promotion and application of SHBG detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Construction of expression vector pET30a(+)-SHBG(151-654bp)
[0023] 1.1 PCR to obtain SHBG (151-654bp)
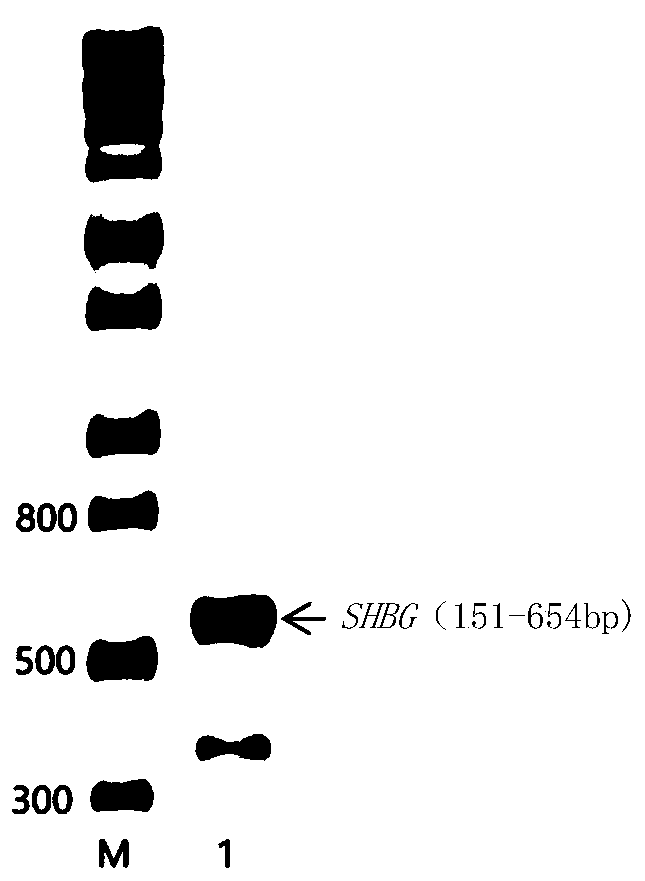
[0024] Total RNA was extracted from 7702 human hepatocytes using the RNA extraction kit from TaKaRa Company, and the cDNA obtained by reverse transcription was used as a template to amplify the target fragment. PCR reaction system: 0.4ul upstream and downstream primers, 2.0ul template, PCR PfuSuperMix 10ul, wxya 2 O 7.2ul; pre-denaturation at 95°C for 1min, denaturation at 95°C for 20s, annealing at 68°C for 20s, extension at 72°C for 45s, 40 cycles, extension at 72°C for 5min, the PCR product was identified by 1.2% agarose gel electrophoresis, and the target item was cut out Band position for agarose gel recovery (OMEGA). Depend on figure 1 It can be seen that there is a clear bright band at about 500bp, which is consistent with the expected size.
[0025] Upstream primer: AAA GGATCC CCAGGACAAGAGCCTATCGC (the underline is BamH I restriction enzyme site)
[0...
Embodiment 2
[0033] Prokaryotic expression of N-terminal 51-218aa of recombinant SHBG protein
[0034] 2.1 Transform the pET30a(+)-SHBG(151-654bp) recombinant plasmid into the expression host Escherichia coli BL21(DE3) to obtain a recombinant engineering strain, namely BL21-SHBG(151-654bp).
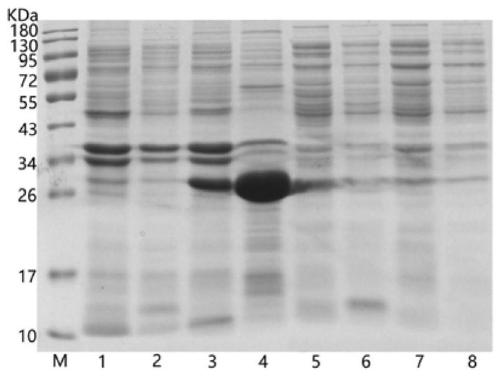
[0035] 2.2 Induced expression: The recombinant engineered strain BL21-SHBG (151-654bp) was picked and inoculated in LB liquid medium (containing 25ug / mL kanamycin), overnight at 37°C and 200rpm. The next day, inoculate 10% of the inoculum into fresh LB liquid medium (containing 25ug / mL kanamycin), shake and culture at 37°C and 200rpm for about 2.5h-3h, add IPTG with a final concentration of 1mM to induce, At the same time, the plasmid pET30a(+) negative control was carried out, and induced for 4.5 hours at 37° C. and 200 rpm. The bacteria were centrifuged at 2500g for 30min at 4°C to collect the lower layer of bacteria. Resuspend the bacteria with an appropriate amount of PBS buffer, carry out 12% c...
Embodiment 3
[0037] Purification of N-terminal 51-218aa of recombinant SHBG protein
[0038] 3.1 Inclusion body treatment: Resuspend the bacteria with an appropriate amount of pre-cooled PBS buffer, sonicate in an ice bath (380W, 60min, 5s on, 6s off), centrifuge at 12000rpm for 10min at 4°C, and collect the precipitated inclusion bodies. Wash the inclusion bodies with an appropriate amount of washing buffer (20mM Tris-HCl, pH 7.9, 500mM NaCl, 10mM imidazole, 1M urea), centrifuge at 12000rpm at 4°C for 10min, and repeat the operation several times until the inclusion bodies are cleaned. Add equilibration buffer (20mM Tris-HCl, pH 7.9, 500mM NaCl, 10mM imidazole, 6M urea) to dissolve the inclusion bodies, and after 1 hour on ice, centrifuge at 10000g for 20min at 4°C to collect the supernatant.
[0039]3.2 Chromatography column: Load the supernatant protein solution into a Ni agarose gel chromatography column and place it in a shaker at 4°C for 2 hours for adsorption. Use 15 times column v...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com