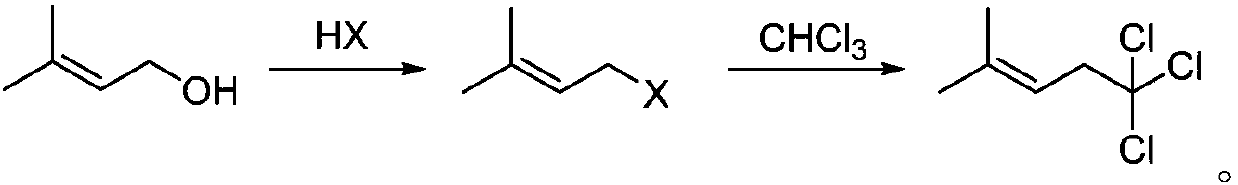
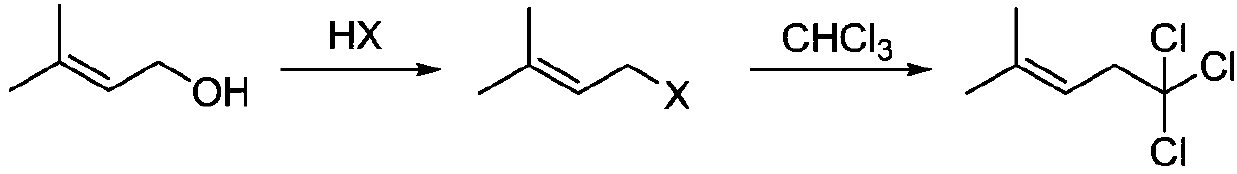
Synthesis method of 5,5,5-trichloro-2-methyl-2-pentene
A synthetic method, methyl technology, applied in chemical instruments and methods, preparation of halogenated hydrocarbons, organic chemistry, etc., can solve the problems of high reaction cost and high environmental pollution, reduce corrosion, reduce pollution, and increase chemical yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add 50.0 g of 3-methyl-2-buten-1-ol into a 500.0 ml four-neck flask, drop the temperature to 0°C, add 88.4 g of 36% hydrochloric acid dropwise, stir at room temperature for 1 hour after the dropwise addition, and let stand to separate layers. The organic layer was dried over anhydrous sodium sulfate, and 52.0 g of an intermediate product was distilled off under reduced pressure.
[0018] In a 250.0 milliliter four-necked flask, add 20.0 grams of intermediate product, 100 milliliters of tetrahydrofuran, 10.7 grams of calcium oxide, 45.7 grams of chloroform, add 9.2 grams of sodium hydroxide under 0 degree stirring state, after gas chromatography tracking analysis, after the completion of the reaction, filter, the mother liquor After the solvent was removed under pressure, 28.1 g of the product 5,5,5-trichloro-2-methyl-2-pentene was distilled out, with a yield of 78%.
Embodiment 2
[0020] Add 50.0 g of 3-methyl-2-buten-1-ol into a 500.0 ml four-neck flask, drop the temperature to 0°C, add 88.4 g of 36% hydrochloric acid dropwise, stir at room temperature for 1 hour after the dropwise addition, and let stand to separate layers. The organic layer was dried over anhydrous sodium sulfate, and 52.0 g of an intermediate product was distilled off under reduced pressure.
[0021] Add 20.0 grams of intermediate product, 100 milliliters of N,N-dimethylformamide, 10.7 grams of calcium oxide, and 45.7 grams of chloroform into a 250.0 milliliter four-neck flask, add 12.8 grams of potassium hydroxide under stirring at 0 degrees, and follow up the analysis by gas chromatography After the reaction was completed, the mother liquor was filtered and the solvent was removed under reduced pressure, and 29.2 g of the product 5,5,5-trichloro-2-methyl-2-pentene was distilled out, with a yield of 81%.
Embodiment 3
[0023] Add 50.0 grams of 3-methyl-2-buten-1-ol into a 500.0 ml four-neck flask, drop the temperature to 0°C and add 140.0 grams of 40% hydrobromic acid dropwise, stir at room temperature for 1 hour after the dropwise addition, and let stand to separate layers . The organic layer was dried over anhydrous sodium sulfate, and 60.0 g of the intermediate product was distilled off under reduced pressure.
[0024] In a 250.0 milliliter four-necked flask, add 20.0 grams of intermediate product, 100 milliliters of tetrahydrofuran, 15.0 grams of calcium chloride, 45.7 grams of chloroform, add 6.5 grams of sodium hydride under stirring state at 0 degrees, after gas chromatography tracking analysis, filter, and the mother liquor reduces After the solvent was removed under pressure, 28.8 g of the product 5,5,5-trichloro-2-methyl-2-pentene was distilled out, with a yield of 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com