Eltrombopag oral suspension and preparation method thereof
A suspension and solution technology, applied in the fields of blood diseases, liquid delivery, pharmaceutical formulations, etc., can solve the problems that oral solid preparations are unfavorable for children and the elderly to swallow, the production cost of liquid capsules is high, and the economic burden of patients is increased. Physical and chemical stability, good physical and chemical compatibility, and good quality indicators
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
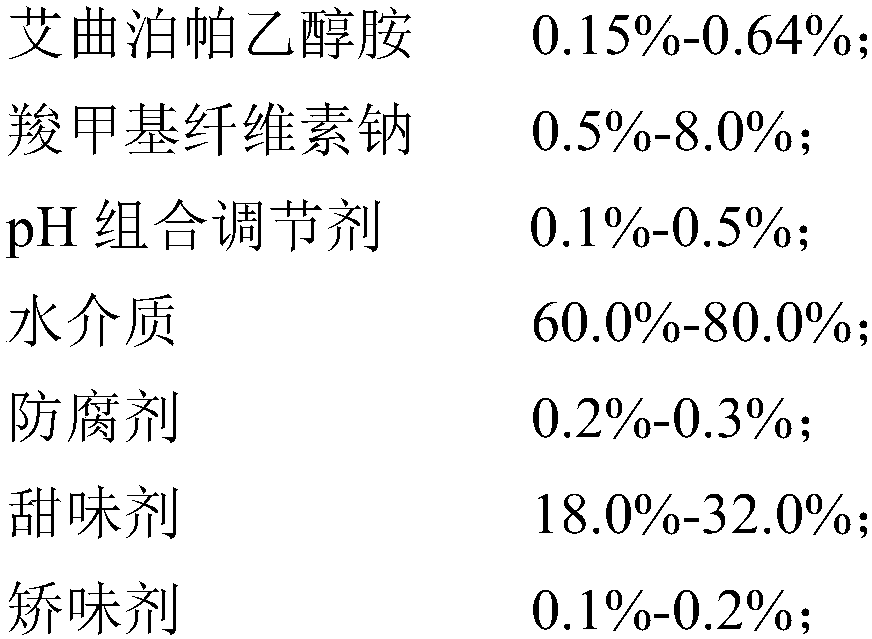
[0049] (1) Preparation prescription (1000mL in total):
[0050]
[0051]
[0052] (2) Preparation process:
[0053] 1) Disperse sodium carboxymethyl cellulose in 800mL of water medium, heat to 80-100°C under agitation to swell and clarify, and cool to room temperature to obtain solution I.
[0054] 2) Add Eltrombopag ethanolamine (Eltrombopag particle size D90 is 20-50 μm) to solution I. Homogenization speed 3000-6000rpm, disperse for 5-10min, mix and flocculate to obtain suspension II;
[0055] 3) adding citric acid and sodium citrate to the suspension II to adjust the pH value to 6.0-6.8 to obtain the suspension III;
[0056] 4) Add preservative sodium benzoate, sweetener mannitol and flavoring agent sweet orange powder essence to suspension III, stir for 10 minutes under the condition of 30 Hz, add water to 1000 mL after dissolving, and obtain Eltrombopag oral suspension liquid.
Embodiment 2
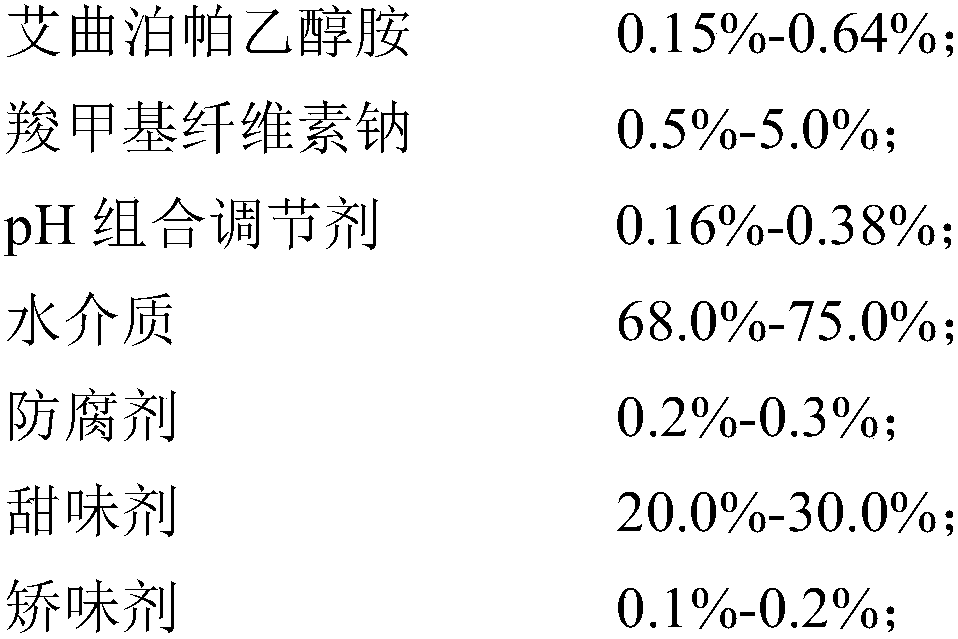
[0058] (1) Preparation prescription (1000ml made in total):
[0059]
[0060] (2) Preparation process:
[0061] 1) Disperse sodium carboxymethyl cellulose in 800mL of water medium, heat to 80-100°C under agitation to swell and clarify, and cool to room temperature to obtain solution I.
[0062] 2) Add Eltrombopag ethanolamine (Eltrombopag particle size D90 is 10-40 μm) to solution I, disperse for 5-10 min at a homogenizing speed of 3000-6000 rpm, mix well, and flocculate to obtain suspension II;
[0063] 3) adding citric acid and sodium citrate to the suspension II to adjust the pH value to 6.0-6.8 to obtain the suspension III;
[0064] 4) Add preservative sodium benzoate, sweetener mannitol and flavoring agent sweet orange powder essence to suspension III, stir at 30 Hz for 10 min, add water to 1000 mL after dissolving, and obtain Eltrombopag oral suspension.
Embodiment 3
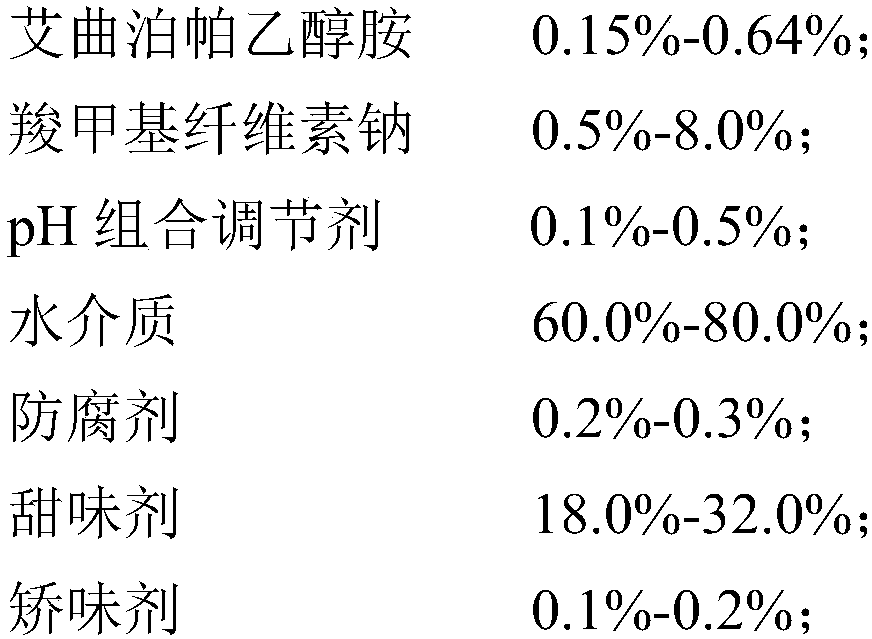
[0066] (1) Preparation prescription (1000mL in total):
[0067]
[0068] (2) Preparation process:
[0069] 1) Disperse sodium carboxymethyl cellulose in 800mL of water medium, heat to 80-100°C under agitation to swell and clarify, and cool to room temperature to obtain solution I.
[0070] 2) Add Eltrombopag ethanolamine (Eltrombopag particle size D90 is 50-90 μm) to solution I, disperse at a homogenizing speed of 3000-6000 rpm for 5-10 minutes, mix and flocculate to obtain suspension II;
[0071] 3) adding citric acid and sodium citrate to the suspension II to adjust the pH value to 6.0-6.8 to obtain the suspension III;
[0072] 4) Add preservative sodium benzoate, sweetener mannitol and flavoring agent sweet orange powder essence to suspension III, stir at 30 Hz for 10 min, add water to 1000 mL after dissolving, and obtain Eltrombopag oral suspension.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com