A chiral α-quaternary carbon-α-hydroxyl-β-amino ketone derivative and its synthesis method
A synthetic method and technology of aminoketones, applied in chemical instruments and methods, preparation of steroids, cyanide reactions, etc., can solve the problem of the inability to realize the selectivity control of α-hydroxy-β-aminoketone products and the total yield of the reaction Low cost, long synthetic route and other issues, to achieve the effect of efficient atom economy, high atom economy, and short steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0118]
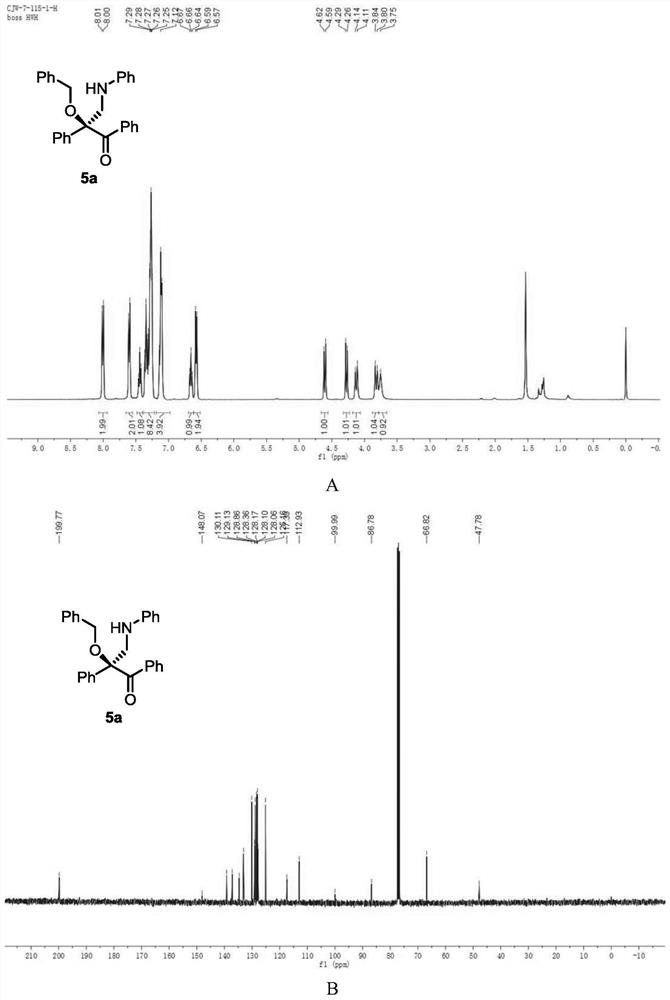
[0119] Add metal catalyst (1mol%), CPA (5mol%) and activated Molecular sieves (100 mg) and sealed with a rubber revolving plug. Ventilate, and replace the air in the system with nitrogen. Dichloromethane (1 mL) was added to the test tube via syringe and stirred at -10°C. After that, dissolve alcohol (1equiv), diazo compound (1equiv), 1,3,5-triaryl-1,3,5-triazine (0.33equiv) in 1mL of dichloromethane and slowly add to the reaction solvent with a peristaltic pump In, the dropping rate is 1mL / h. After the dropwise addition, the stirring reaction was continued until the diazo compound was completely consumed. Stop the reaction, use a capillary to take a small amount of the reaction solution, purify it by TLC, and use it for chiral HPLC analysis, and finally purify it by column chromatography (silica gel H, eluent: EA:PE = 1:200~1:50) to obtain a white solid product 5a. Yield 81%, ee% = 78%. 1 H NMR (400MHz, CDCl 3 )δ8.00 (d,2H),7.60(d,J=7.7Hz,2H),7.44(t,J=7.4Hz...
Embodiment 2
[0121]
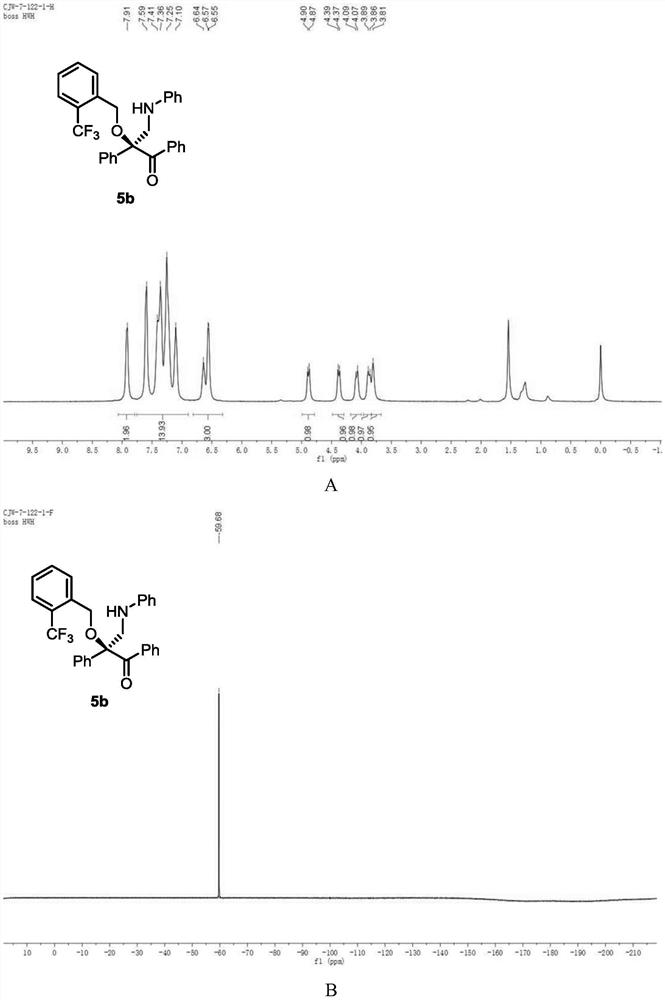
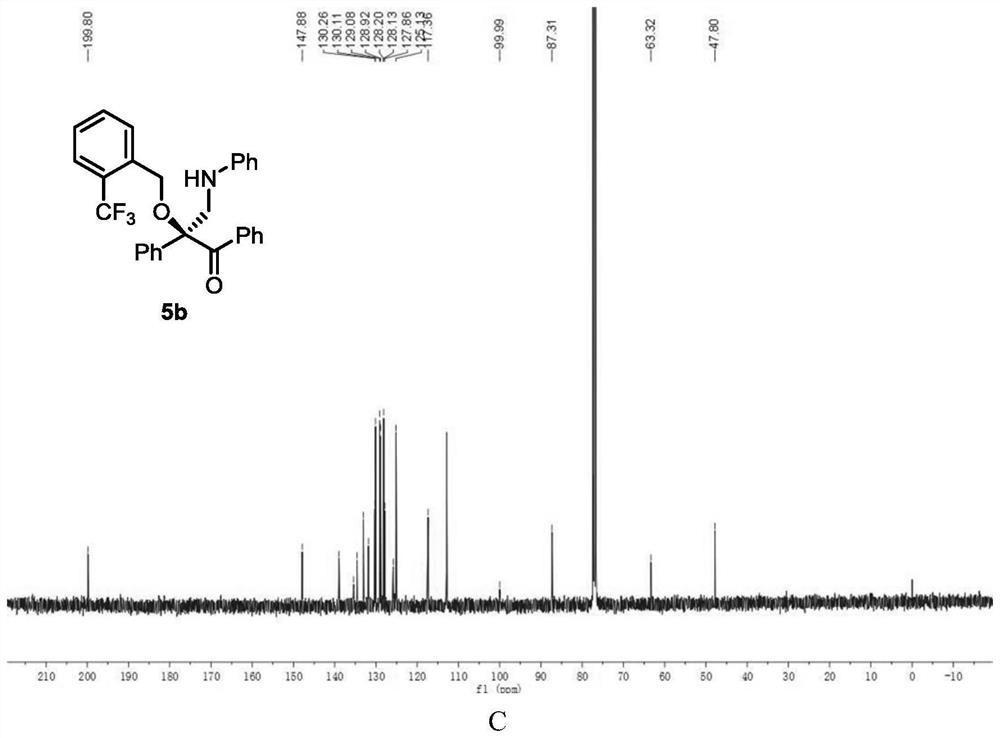
[0122] Add metal catalyst (1mol%), CPA (5mol%) and activated Molecular sieves (100 mg) and sealed with a rubber revolving plug. Ventilate, and replace the air in the system with nitrogen. Dichloromethane (1 mL) was added to the test tube via syringe and stirred at -10°C. After that, dissolve alcohol (1equiv), diazo compound (1equiv), 1,3,5-triaryl-1,3,5-triazine (0.33equiv) in 1mL of dichloromethane and slowly add to the reaction solvent with a peristaltic pump In, the dropping rate is 1mL / h. After the dropwise addition, the stirring reaction was continued until the diazo compound was completely consumed. Stop the reaction, use a capillary to take a small amount of the reaction solution, purify it by TLC, and use it for chiral HPLC analysis, and finally purify it by column chromatography (silica gel H, eluent: EA:PE = 1:200~1:50) to obtain a white solid product 5b. Yield 81%, ee% = 94%. 1 H NMR (400MHz, CDCl 3 )δ7.91 (s,2H),7.69–6.99(m,14H),6.70–6.47(m,3H),...
Embodiment 3
[0124]
[0125] Add metal catalyst (1mol%), CPA (5mol%) and activated Molecular sieves (100 mg) and sealed with a rubber revolving plug. Ventilate, and replace the air in the system with nitrogen. Dichloromethane (1 mL) was added to the test tube via syringe and stirred at -10°C. After that, dissolve alcohol (1equiv), diazo compound (1equiv), 1,3,5-triaryl-1,3,5-triazine (0.33equiv) in 1mL of dichloromethane and slowly add to the reaction solvent with a peristaltic pump In, the dropping rate is 1mL / h. After the dropwise addition, the stirring reaction was continued until the diazo compound was completely consumed. Stop the reaction, use a capillary to take a small amount of the reaction solution, purify it by TLC, and use it for chiral HPLC analysis, and finally purify it by column chromatography (silica gel H, eluent: EA:PE = 1:200~1:50) to obtain a white solid product 5c. Yield 76%, ee% = 90%. 1 H NMR (400MHz, CDCl 3 )δ8.03 (d, J = 7.7Hz, 2H), 7.59 (d, J = 7.6Hz, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com