Preparation method and applications of impregnation type mono-atomic iron-carbon layer modified nickel based or cobalt based composite material electrode
A composite material and impregnated technology, which is applied in the field of electrochemistry, can solve problems that have not yet been discovered, and achieve the effects of easy industrial implementation, cheap and easy-to-obtain raw materials, and low electrocatalytic decomposition of water for oxygen evolution reaction overpotential
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
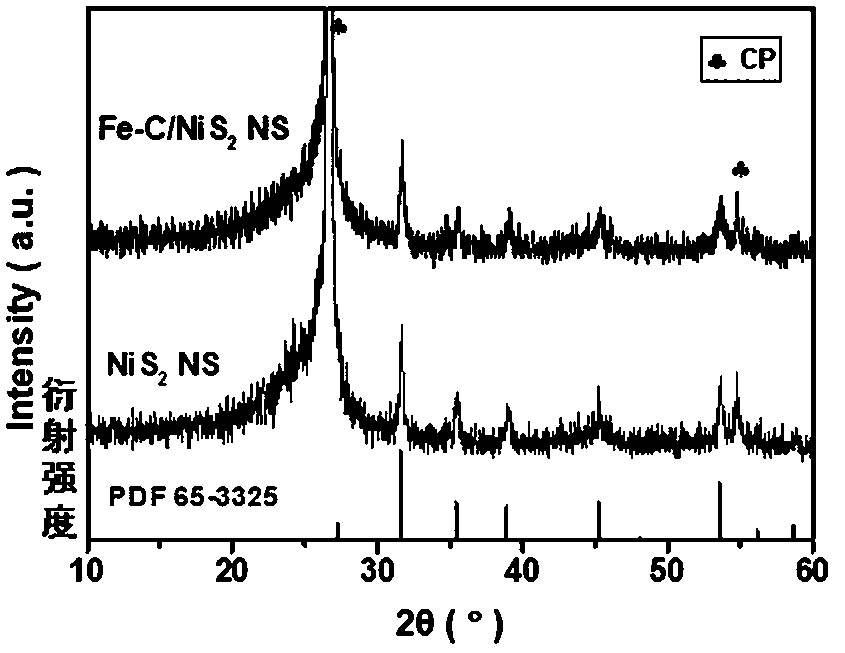
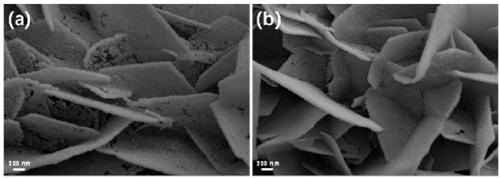
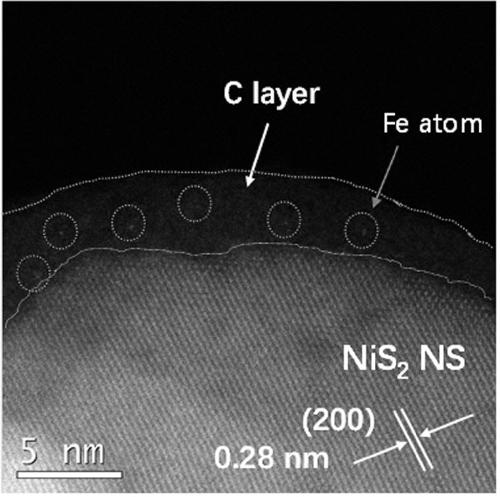
[0031] The preparation of the iron-carbon (Fe-C) electrode adopts the impregnation-calcination method: Weigh 0.1 g of tannic acid in a 50 mL beaker, add 5 mL of ethanol solution to it, stir magnetically until it is completely dissolved, and form solution A; Weigh 0.1 g of ferric nitrate nonahydrate and dissolve it in 5 mL of ethanol solution to form solution B; slowly add the obtained clear solution B to solution A dropwise, and stir magnetically at room temperature for 2 h to form colloidal solution C. Take a size of about 1×2 cm 2 The carbon cloth (CC or carbon paper CP or conductive glass FTO or stainless steel mesh SSM) was immersed in colloidal solution C for about 1 h, taken out, put into an oven, and dried at 60 °C for 6 h. Then transfer the impregnated carbon cloth (CC or carbon paper CP or conductive glass FTO or stainless steel mesh SSM) into a semi-closed porcelain boat, and then transfer it to a temperature-programmed tube furnace. The tube furnace was heated to 3...
Embodiment 2
[0033] The preparation of the iron-carbon (Fe-C) electrode adopts the impregnation-calcination method: Weigh 0.1 g of tannic acid in a 50 mL beaker, add 5 mL of ethanol solution to it, stir magnetically until it is completely dissolved, and form solution A; Weigh 0.1 g of ferric nitrate nonahydrate and dissolve it in 5 mL of ethanol solution to form solution B; slowly add the obtained clear solution B to solution A dropwise, and stir magnetically at room temperature for 2 h to form colloidal solution C. Take a size of about 1×2 cm 2 The carbon cloth (CC or carbon paper CP or conductive glass FTO or stainless steel mesh SSM) was immersed in colloidal solution C for about 1 h, taken out, put into an oven, and dried at 60 °C for 6 h. Then transfer the impregnated carbon cloth (CC or carbon paper CP or conductive glass FTO or stainless steel mesh SSM) into a semi-closed porcelain boat, and then transfer it to a temperature-programmed tube furnace. The tube furnace was heated to 4...
Embodiment 3
[0035]The preparation of the iron-carbon (Fe-C) electrode adopts the impregnation-calcination method: Weigh 0.1 g of tannic acid in a 50 mL beaker, add 5 mL of ethanol solution to it, stir magnetically until it is completely dissolved, and form solution A; Weigh 0.1 g of ferric nitrate nonahydrate and dissolve it in 5 mL of ethanol solution to form solution B; slowly add the obtained clear solution B to solution A dropwise, and stir magnetically at room temperature for 2 h to form colloidal solution C. Take a size of about 1×2 cm 2 The carbon cloth (CC or carbon paper CP or conductive glass FTO or stainless steel mesh SSM) was immersed in colloidal solution C for about 1 h, taken out, put into an oven, and dried at 60 °C for 6 h. Then transfer the impregnated carbon cloth (CC or carbon paper CP or conductive glass FTO or stainless steel mesh SSM) into a semi-closed porcelain boat, and then transfer it to a temperature-programmed tube furnace. The tube furnace was heated to 50...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com