Fluorosulfonate-based compound preparation method
A fluorosulfonic acid ester and compound technology, which is applied in the field of preparation of fluorosulfonic acid ester compounds, can solve the problems of unfavorable mass production and green environmental protection concept, affecting application effect, and high water content of additives, and achieves improvement of charge-discharge performance and cycle. The effect of the number of times, the three wastes is less, and the equipment investment is small
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
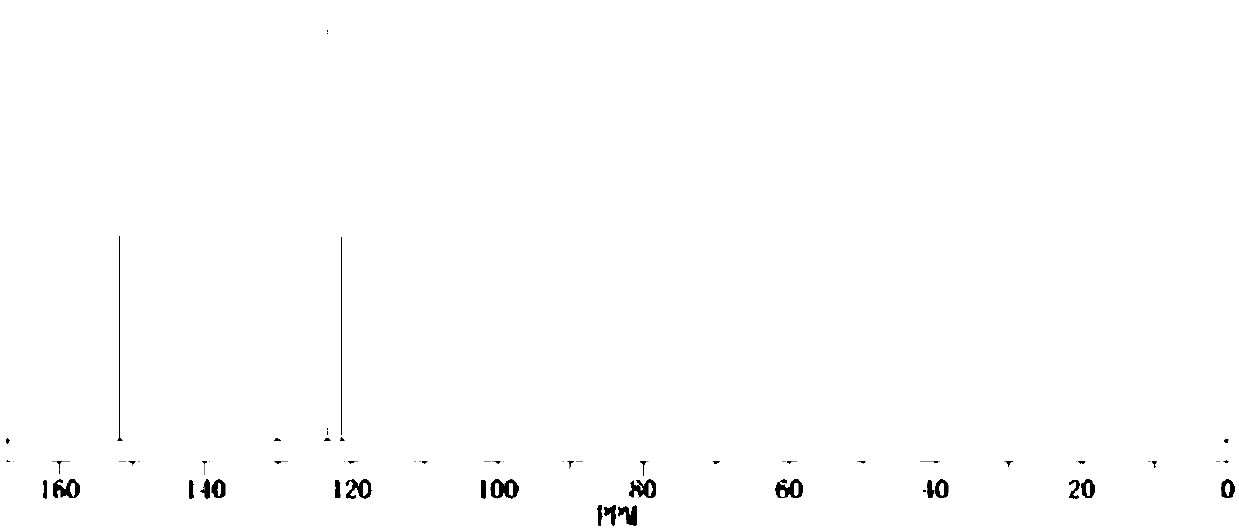
[0023] Preparation of fluorosulfonyloxybenzene:
[0024] Chemical formula:
[0025] Take 1mmol of phenol, 1.1mmol of triethylamine, and 14mL of dioxane into the reaction vessel, stir at room temperature for 1h, pass 1mmol of sulfuryl fluoride gas into it, control the reaction pressure of the system to 7atm, and the reaction temperature to 50°C, and react for 24h. Then add hydrochloric acid aqueous solution to acidify for 20min, spin the solvent under reduced pressure, separate and purify by silica gel column chromatography, and select petroleum ether (60°C) and ethyl acetate with a volume ratio of 0.1:1 as the eluent to obtain 158.5 mg of fluorosulfonyl oxide Benzene.
[0026] The density of the detected fluorosulfonyloxybenzene is 1.42g / cm 3 , the boiling point is 221.3°C 760mmHg, the purity is 99.5%, the water content is 30ppm, the acid value is 36ppm, and the yield is 89.97%.
Embodiment 2
[0028] Preparation of fluorosulfonyloxyethane:
[0029] Chemical formula:
[0030] Take 1mmol of ethanol, 1.5mmol of triethylamine, and 8mL of dioxane into the reaction vessel, stir at room temperature for 2 hours, pass 1.1mmol of sulfuryl fluoride gas into it, control the reaction pressure of the system to 8 atm, and the reaction temperature to 45°C, and react for 20 hours , and then add hydrochloric acid aqueous solution to acidify for 30 minutes, spin the solvent under reduced pressure, separate and purify by silica gel column chromatography, and select petroleum ether (70°C) and ethyl acetate with a volume ratio of 0.2:1 as the eluent to obtain 119.15 mg of fluorosulfonyl Oxyethane.
[0031] The density of the detected fluorosulfonyloxyethane is 1.35g / cm 3 , the boiling point is 117.2°C 760mmHg, the purity is 99.7%, the water content is 27ppm, the acid value is 32ppm, and the yield is 93%.
Embodiment 3
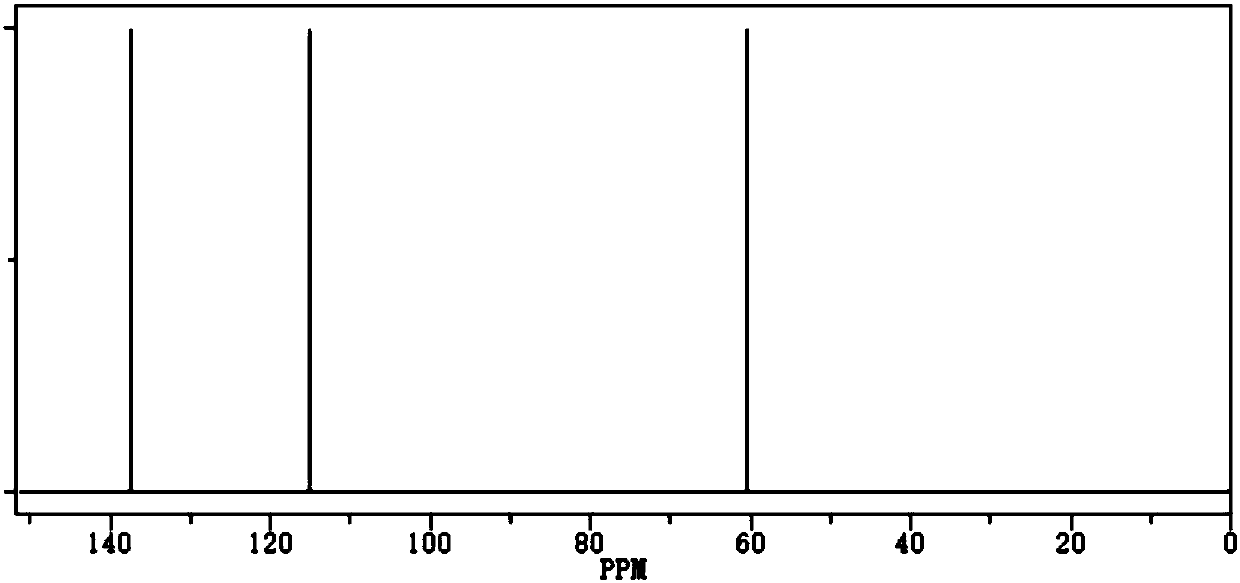
[0033] Preparation of allyl fluorosulfonate:
[0034] Chemical formula:
[0035] Take 1mmol of allyl alcohol, 1.2mmol of triethylamine, and 10mL of dioxane into the reaction vessel, stir at room temperature for 1.5h, and pass 1.05mmol of sulfuryl fluoride gas into it, control the reaction pressure of the system to 9atm, and the reaction temperature to 40°C , reacted for 15h, then added hydrochloric acid aqueous solution to acidify for 25min, spin-dried the solvent under reduced pressure, separated and purified by silica gel column chromatography, and selected petroleum ether (80°C) and ethyl acetate with a volume ratio of 0.1:1 as the eluent to obtain 128.3mg Allyl Fluorosulfonate.
[0036] The density of the obtained allyl fluorosulfonate is 1.29g / cm 3 , the boiling point is 210°C 760mmHg, the purity is 99.5%, the water content is 23ppm, the acid value is 25ppm, and the yield is 91.64%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com