Preparation and application of non-noble metal nitrogen-doped hollow carbon nanotube electrocatalyst
A non-precious metal, electrocatalyst technology, applied in the direction of nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., to achieve the effect of a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
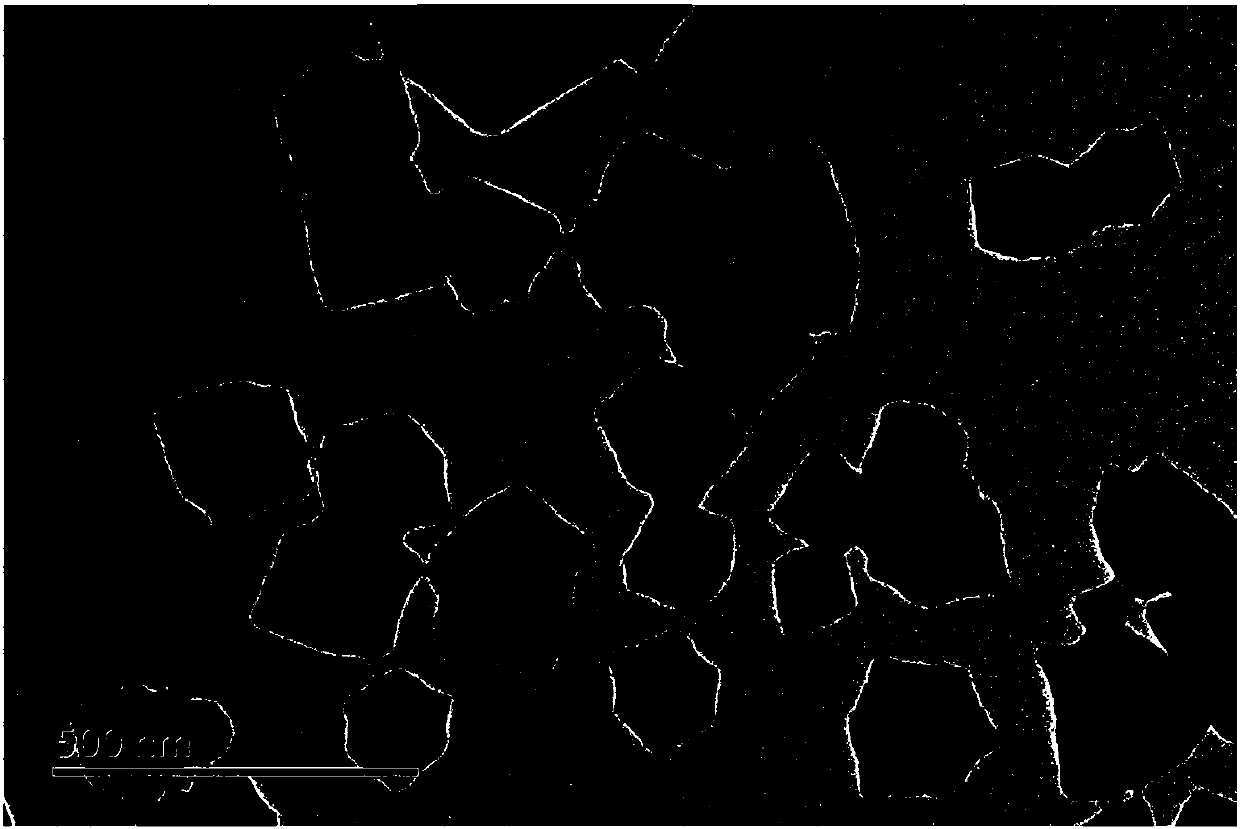
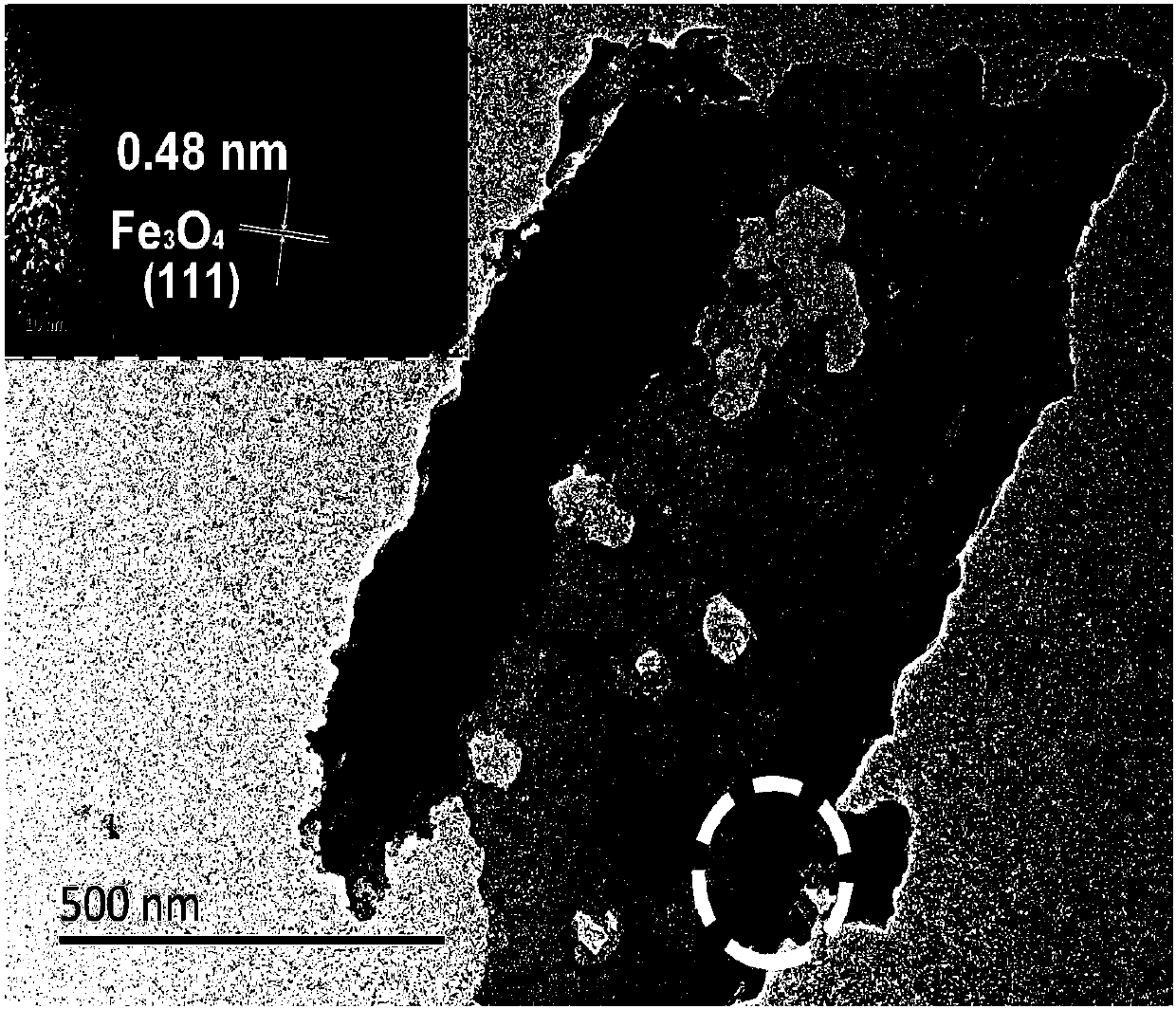
Image
Examples
Embodiment 1
[0026] 1. Using MIL-101(Fe) as the precursor, first add 1.328g terephthalic acid and 2.168g to 394ml DMF to prepare 62.5mg FeCl 3 ·6H 2 O, after stirring and reacting at 150° C. for 24 hours in a single-necked flask, filter, centrifuge and dry the resulting precipitate to obtain about 0.8 g of light pink MIL-101(Fe) powder.
[0027] 2. Immerse the above-mentioned light pink powder in aniline monomer at a concentration of 0.12g / ml, stir at room temperature for 2 days, filter and wash with a small amount of ether, and dry in an oven at 60°C to obtain a khaki MIL-ANI (aniline) compound The product is about 0.9g.
[0028] 3. Dissolve the MIL-ANI complex in 1M HCl solution at a concentration of 0.04g / ml, and add 0.18g / ml ammonium persulfate / 1M HCl solution dropwise with stirring at a low temperature below 4°C until the solution becomes After dark green, the polymerization reaction was continued for 4 hours, filtered and washed with water until neutral, and dried to obtain about 0...
Embodiment 2
[0032] The difference between this example and Example 1 is that the immersion time of MIL-101(Fe) powder in the aniline monomer is 0.5 days (12 hours), and the polymerization time of aniline is 20 hours, and the conditions of the two pyrolysis are respectively 800°C for 2 hours and 1000°C for 1 hour. The resulting catalyst is designated C-MIL-PANI-2.
[0033] Figure 5 N for C-MIL-PANI-2 catalyst 2 According to the physical adsorption-desorption curve, the BET specific surface area is 862.826m 2 g -1 . Figure 6 It is the performance diagram of the full cell when C-MIL-PANI-2 is used as the cathode catalyst of the proton exchange membrane fuel cell. It can be seen that its highest power density can reach 150mW / cm 2 .
Embodiment 3
[0035] The difference between this example and Example 1 is that the self-made ZIF-8 white powder and MIL-101(Fe) powder are ground and mixed with the same amount of substances, and then impregnated in the aniline monomer together. The resulting catalyst is designated as C-MIL / ZIF-PANI. Wherein, the preparation method of ZIF-8 is as follows:
[0036] In a 250ml single-necked flask, add 125mL ethanol, 1.1900g Zn(NO 3 ) 2 ·6H 2 0 and 1.6405g 2-methylimidazole, stirred and reacted at room temperature for 24 hours, filtered and centrifuged, washed and dried to obtain about 0.4g of ZIF-8 white powder.
[0037] Figure 7 The performance diagram of the full cell when the C-MIL / ZIF-PANI prepared for Example 3 is used as the cathode catalyst of the proton exchange membrane fuel cell shows that the power density of the cell decreases to a certain extent as the operating time of the cell increases.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com