Silanol organic compound and preparation method thereof
A technology of organic compounds and silanols, applied in the direction of silicon organic compounds, etc., can solve the problems of expensive chlorosilane raw materials, difficulty in industrialized production, and difficult to handle organic waste, etc., and achieve the effect of cheap catalysts, wide application range, and clean oxidants
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
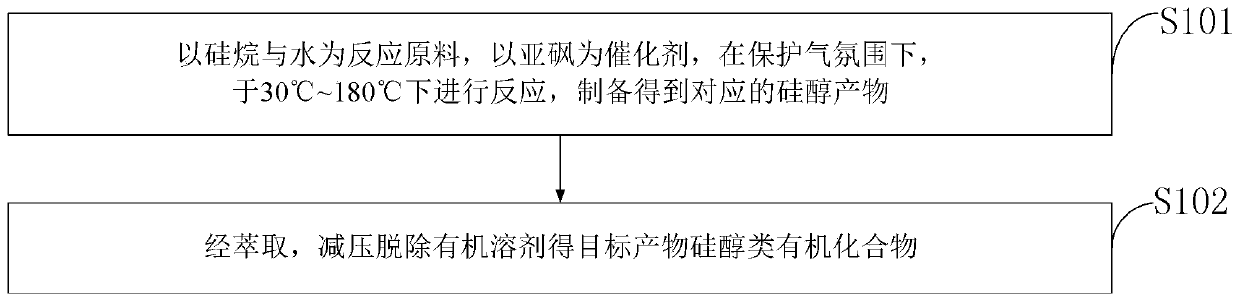
[0037] Such as figure 1 As shown, the preparation method of the silanol organic compound provided by the embodiments of the present invention comprises:
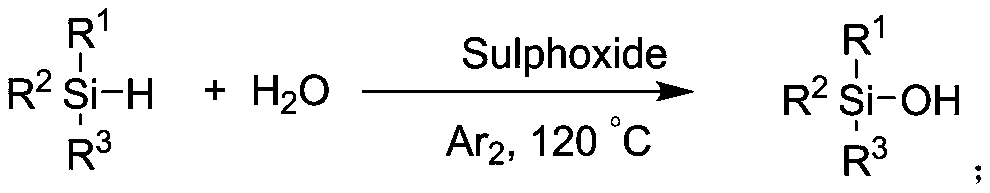
[0038] S101: using silane and water as reaction raw materials, using sulfoxide as a catalyst, and reacting under a protective gas atmosphere at 30° C. to 180° C. to prepare corresponding silanol products.
[0039] S102: After extraction, the organic solvent is removed under reduced pressure to obtain the target product silanol organic compound.
[0040] In the present invention, water is selected from domestic water, and the molar ratio of silane:water is 1:1-500.
[0041] In the present invention, sulfoxide is selected from one of dimethyl sulfoxide, diphenyl sulfoxide, dibenzyl sulfoxide, methylphenyl sulfoxide, benzyl methyl sulfoxide, and tetramethylene sulfoxide. A mixture of one or more sulfoxides, the volume ratio of silane to organic solvent is 1:2-20.
[0042] In the present invention, the protective gas is selec...
Embodiment 1
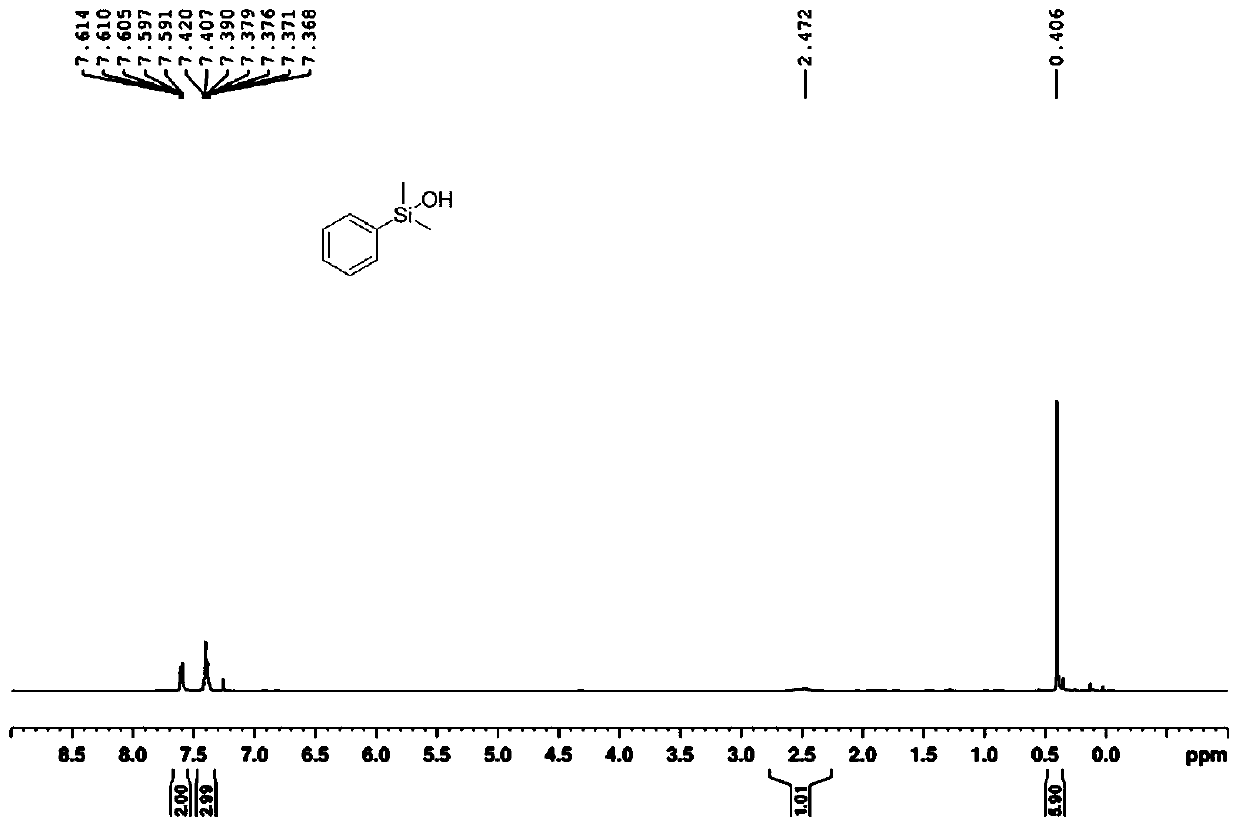
[0054] In a 15ml reaction tube equipped with a magnetic stirring bar, send it to the glove box, add 81.6mg 1,3-dimethylphenylsilane, 2ml dimethyl sulfoxide, 324ul water, under argon atmosphere, temperature The temperature is 120°C, the magnetic stirrer is 800rpm, and the reaction process is detected by TLC. After the silane reaction is complete, the target product is extracted and concentrated. The product is 1,3-dimethylphenylsilanol, a colorless oily liquid, and the yield is 93% (according to silane), 1 H NMR (400MHz, CDCl 3 )δ:7.61-7.59(2H,m),7.42-7.37(3H,m),2.47(1H,br),0.41(6H,s); 13 C NMR (100MHz, CDCl 3 )δ139.2, 133.2, 129.7, 128.0, 0.1, such as figure 2 shown.
Embodiment 2
[0055] Example 2: In a 15ml reaction tube equipped with a magnetic stirring bar, send it to a glove box, add 118.8mg diphenylmethyl silane, 2ml dimethyl sulfoxide, 324ul water, under an argon atmosphere, the temperature The temperature is 120°C, the magnetic stirrer is 800rpm, and the reaction process is detected by TLC. After the silane reaction is complete, the target product is extracted and concentrated. The product is diphenylmethylsilanol, a colorless oily liquid, and the yield is 96% (according to silane meter), 1 H NMR (400MHz, CDCl 3 )δ:7.68-7.59(4H,m),7.47-7.34(6H,m),3.66(1H,br),0.68(3H,s); 13 C NMR (100MHz, CDCl 3 )δ137.1, 133.9, 129.6, 127.8, -1.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com