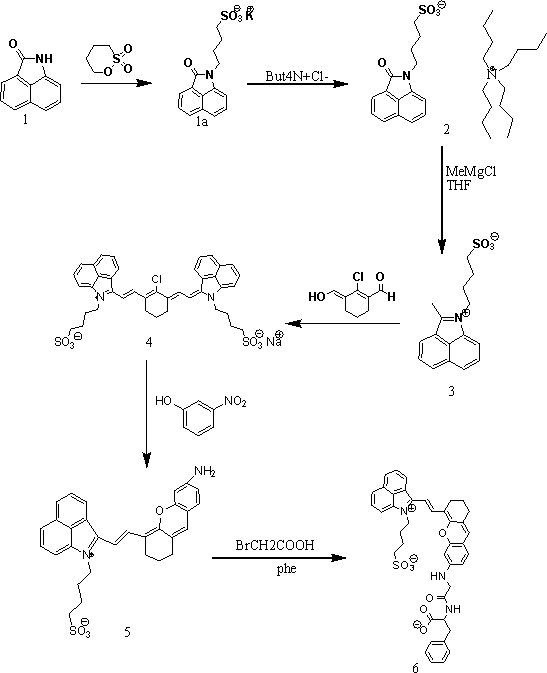
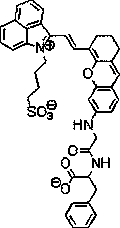
Novel near-infrared fluorescent probe for detecting carboxypeptidase A
A fluorescent probe and near-infrared technology, applied in the field of analytical chemical detection, can solve the problems of carboxypeptidase imaging, etc., and achieve the effect of high synthesis yield, high sensitivity and long emission wavelength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Implementation Example 1: Preparation of Potassium Salt 1a
[0020] Take 1,8-naphtholactam (0.85g, 5mmol), potassium hydroxide (0.56g, 10mmol), dissolve in N-methyl-2-pyrrolidone (10ml), stir at room temperature for 30min, and then add butanol Sultone (0.75g, 5.5mmol) was heated to 90°C for 10h, cooled, and treated with acetone (35ml) to give product 1a (1.65g, 96%). 1 H NMR (400 MHz, DMSO-d6): 8.18 (d, J = 8.1 Hz, 1H), 8.07 (d, J = 8.3 Hz, 1H), 7.81 (t, J = 8.7Hz, 1H), 7.65 (d , J = 8.1 Hz, 1H), 7.55 (t, J = 8.7 Hz, 1H), 7.23 (d, J =7.5Hz, 1H), 3.91 (t, J = 7.2 Hz, 2H), 2.46 (m, 2H ), 1.79 (m, 2H), 1.62 (t, J =7.2 Hz, 2H).
Embodiment 2
[0021] Implementation example 2: the preparation of intermediate product 2
[0022] Take 1a (1.50g, 5mmol), tetrabutylammonium chloride (1.51g, 5.5mmol), dissolve in acetic acid (8ml), stir at 90°C for 0.5h, then add ethyl acetate dropwise, and then filter, Rotary evaporation gave product 2 (2.5 g, 94%) as viscous oil. 1 H NMR(400 MHz, DMSO-d6): 8.01 (t, J = 7.4 Hz, 1H) ,7.66 (t, J = 7.4 Hz, 1H), 7.51(m, 2H) 7.24 (d, J = 7.2 Hz, 1H), 7.17 (d, J = 7.1 Hz, 1H), 6.97 (d, J = 7.1Hz, 1H), 3.93 (t, J = 7.4Hz, 1H), 3.26 (t, J = 8.6 Hz, 8H) , 2.90 (m, 2H), 1.94 (m, 4H), 1.64 (m, 8H), 1.42 (m, 8H), 0.98 (t, J = 8.6 Hz, 12H).
Embodiment 3
[0023] Implementation Example 3: Preparation of Compound 3
[0024] Take product 2 (2.73g, 5mmol) and methylmagnesium chloride, dissolve in anhydrous tetrahydrofuran (20ml), heat to 60°C under nitrogen atmosphere, stir for 1h, then cool, add 1M hydrochloric acid for neutralization, and use ethanol ( 15ml) and diethyl ether (20ml), then cooled to 0°C overnight to give product 3 (2.58 g, 85%). 1 H NMR (D2O): 8.51 (d, J = 8.1 Hz, 1H), 8.43 (d, J = 8.1 Hz, 1H), 8.09 (m, 2H), 7.85 (t, J = 8.5 Hz, 1H), 7.73 (t, J = 8.2 Hz, 1H), 4.51, (t, J = 8.5 Hz, 2H), 3.02 (s, 3H), 2.85 (m, 2H), 2.04 (m, 2H), 1.78 (t, J = 8.2 Hz, 2H). MS (maldi) m / z: calcd. for[C 16 h 19 NO 3 S] + , 304.10; found 304.10.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com