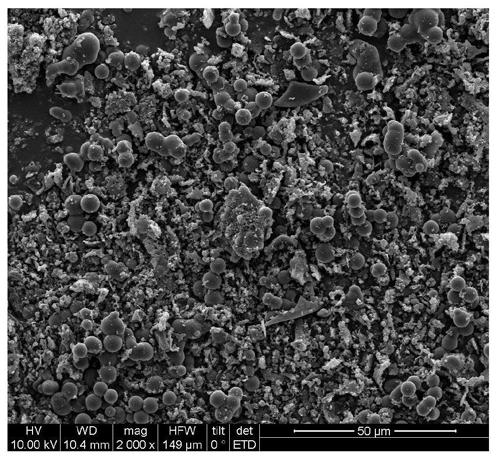
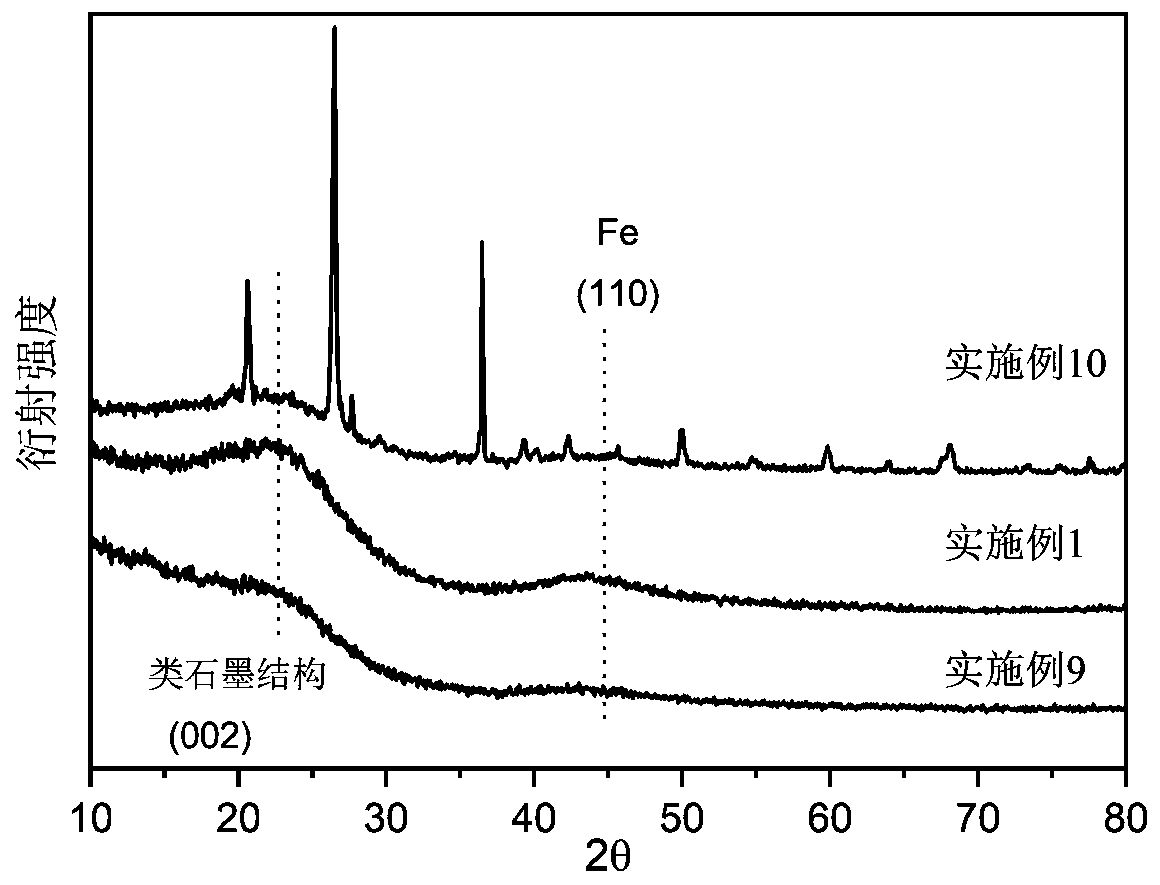
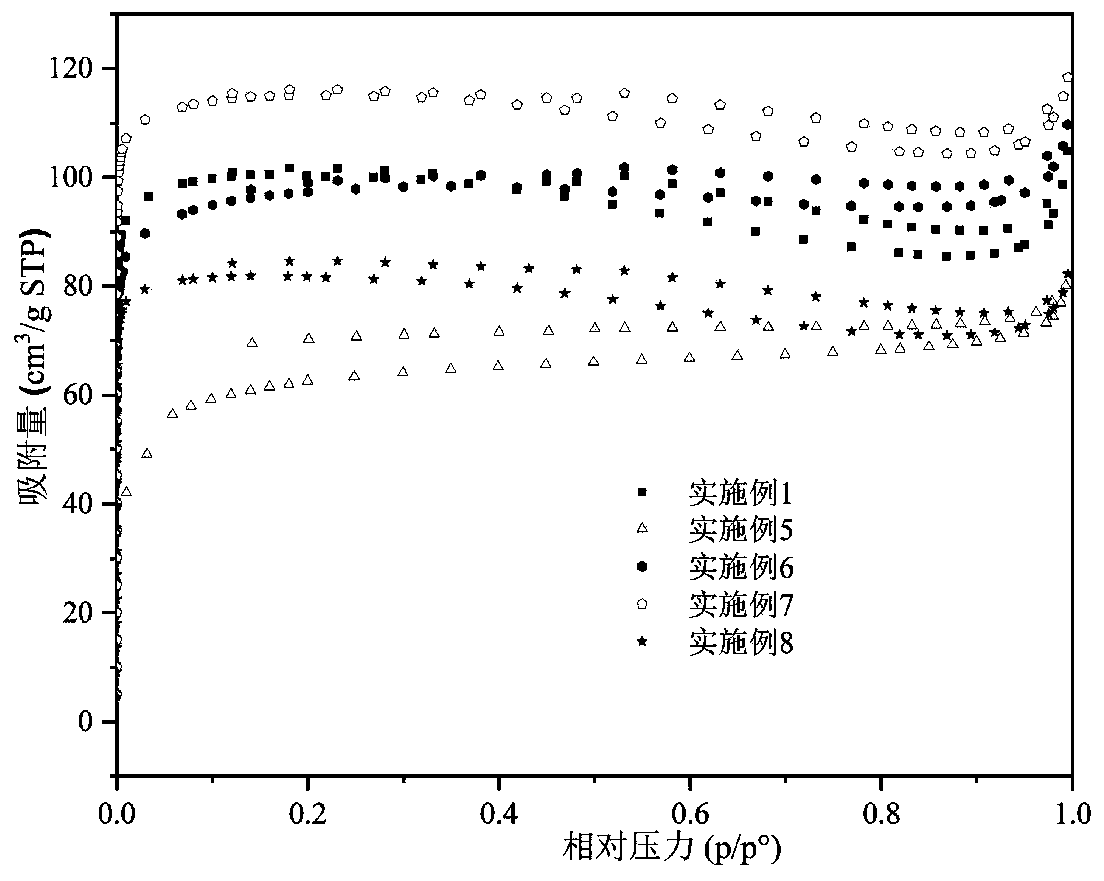
Biochar-loaded zero-valent iron composite material and preparation method thereof
A composite material and biomass material technology, which is applied in the field of biochar-loaded zero-valent iron composite material and its preparation field, can solve the problems of complicated operation, serious environmental pollution and high price, and achieves simple operation, easy availability of raw materials and low cost of raw materials. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The invention provides a method for preparing a biochar-supported zero-valent iron composite material, comprising the following steps:
[0030] (1) mixing the aqueous solution of the biomass material and the iron source, and ultrasonically dispersing to obtain a raw material mixture; the iron source is at least one of iron salt and ferrous salt;
[0031] (2) subjecting the raw material mixture to a hydrothermal reaction to obtain an iron-carbon precursor;
[0032] (3) Calcining the iron-carbon precursor in an inert gas atmosphere to obtain a biochar-loaded zero-valent iron composite material.
[0033] The invention mixes the aqueous solution of the biomass material and the iron source, and disperses through ultrasonic to obtain the raw material mixture.
[0034] In the present invention, the biomass material is preferably at least one of orange peel, orange peel, pomelo peel, lemon peel, orange peel, bamboo powder and cellulose; the pomelo peel is preferably grapefruit...
Embodiment 1
[0062] Washing, drying and pulverizing the pomelo peel in sequence, then passing through a 100-mesh sieve, and taking the part under the sieve to obtain the biomass material;
[0063] The biomass material and the ferric chloride aqueous solution are mixed according to the ratio of solid-liquid ratio of 0.1g:1mL, and the mass ratio of the biomass material and the ferric chloride aqueous solution is 100:10; after mixing, the resulting mixture Ultrasonic dispersion for 120 minutes to obtain a raw material mixture; the power of ultrasonic dispersion is 100W;
[0064] Put the raw material mixture in a polytetrafluoroethylene-lined autoclave for hydrothermal reaction, raise the temperature to 200°C at a heating rate of 1°C / min, and react at 200°C for 2 hours; after the reaction is completed, filter and wash in sequence and freeze-drying to obtain an iron-carbon precursor;
[0065] The iron-carbon precursor was transferred to a tube furnace, heated to 600°C at a rate of 5°C / min in a...
Embodiment 2
[0073] Washing, drying and pulverizing the pomelo peel in sequence, and passing through a 100-mesh sieve to obtain a biomass material;
[0074] The biomass material and the ferric chloride aqueous solution are mixed according to the ratio of solid-liquid ratio of 0.1g:1mL, and the mass ratio of the biomass material and the ferric chloride aqueous solution is 100:5; after mixing, the resulting mixture Ultrasonic dispersion for 120 minutes to obtain a raw material mixture; the power of ultrasonic dispersion is 100W;
[0075] Put the raw material mixture in a polytetrafluoroethylene-lined autoclave for hydrothermal reaction, raise the temperature to 200°C at a heating rate of 1°C / min, and react at 200°C for 2 hours; after the reaction is completed, filter and wash in sequence and freeze-drying to obtain an iron-carbon precursor;
[0076] The iron-carbon precursor was transferred to a tube furnace, heated to 600°C at a rate of 5°C / min in an inert gas atmosphere, and calcined at 6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com