Polyboron phenylalanine compound containing nitroimidazole, as well as preparation method and application thereof
A borophenylalanine and nitroimidazole technology, which is applied in the field of polyboronphenylalanine compounds, can solve the problems of low tumor-specific accumulation ability, poor chemical stability, application limitations and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
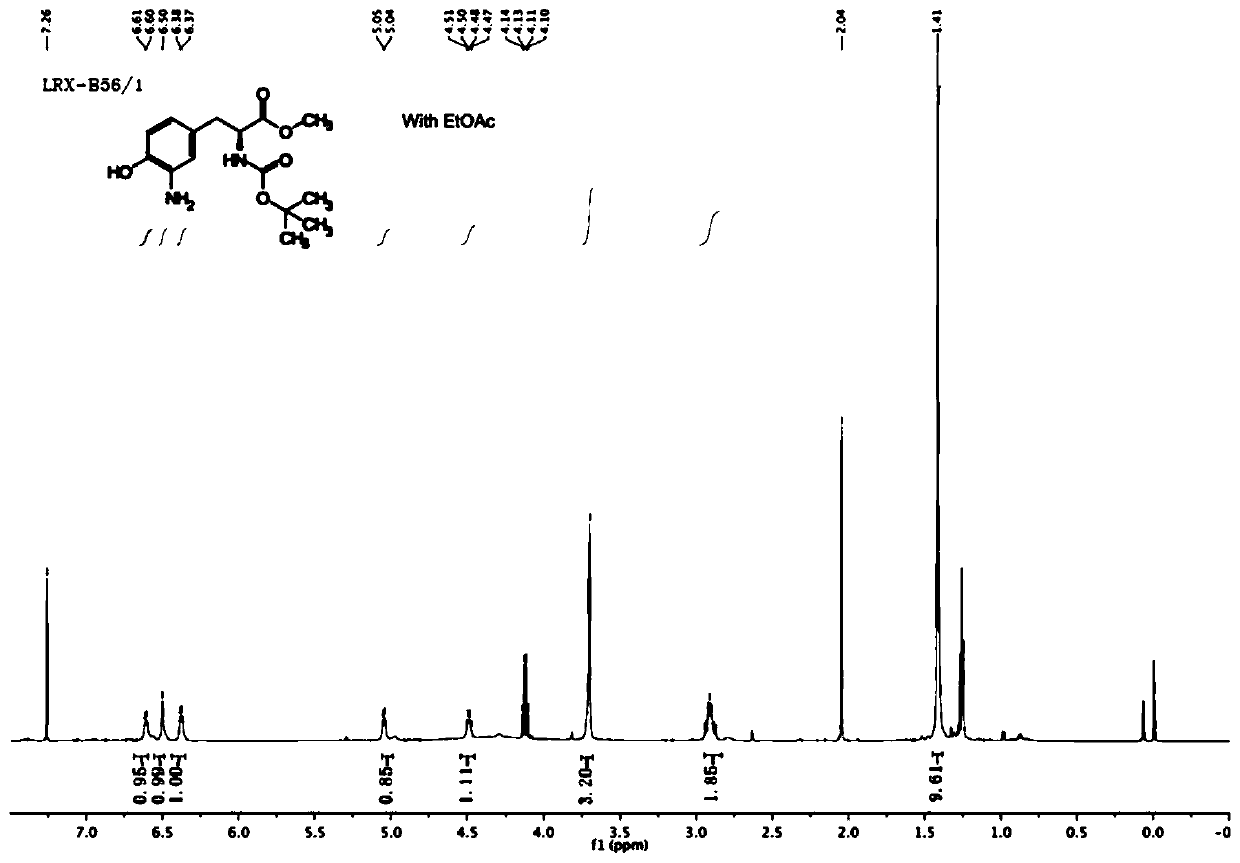
[0096] (1) Preparation of S1 compound
[0097]
[0098] Dissolve 2-nitroimidazole (500mg, 4.42mmol) in 3ml N,N-dimethylformamide, add potassium carbonate (1.22g, 8.84mmol), and add 1,3-dibromopropane ( 1.79ml, 17.69mmol), reacted overnight, TLC monitoring found that the raw material had reacted completely. Extracted three times with ethyl acetate (15ml) and water (15ml), combined the organic phases and distilled the crude product under reduced pressure through column chromatography (petroleum ether: ethyl acetate = 5:1) to obtain a tan solid compound S1, A total of 760mg, yield 73%.
[0099] 1 H NMR (600MHz, Chloroform-d) δ7.21(s,1H),7.17(s,1H),4.63(t,J=6.1Hz,2H),3.38(t,J=5.9Hz,2H),2.45 –2.38(m,2H).
[0100] (2) Preparation of S2 compound
[0101]
[0102] Dissolve 2-nitroimidazole (500mg, 4.42mmol) in 3ml N,N-dimethylformamide, add potassium carbonate (1.22g, 8.84mmol), and add 1-bromo-4-chlorobutyl under stirring Alkanes (1.21ml, 10.44mmol) were reacted overnight...
experiment example
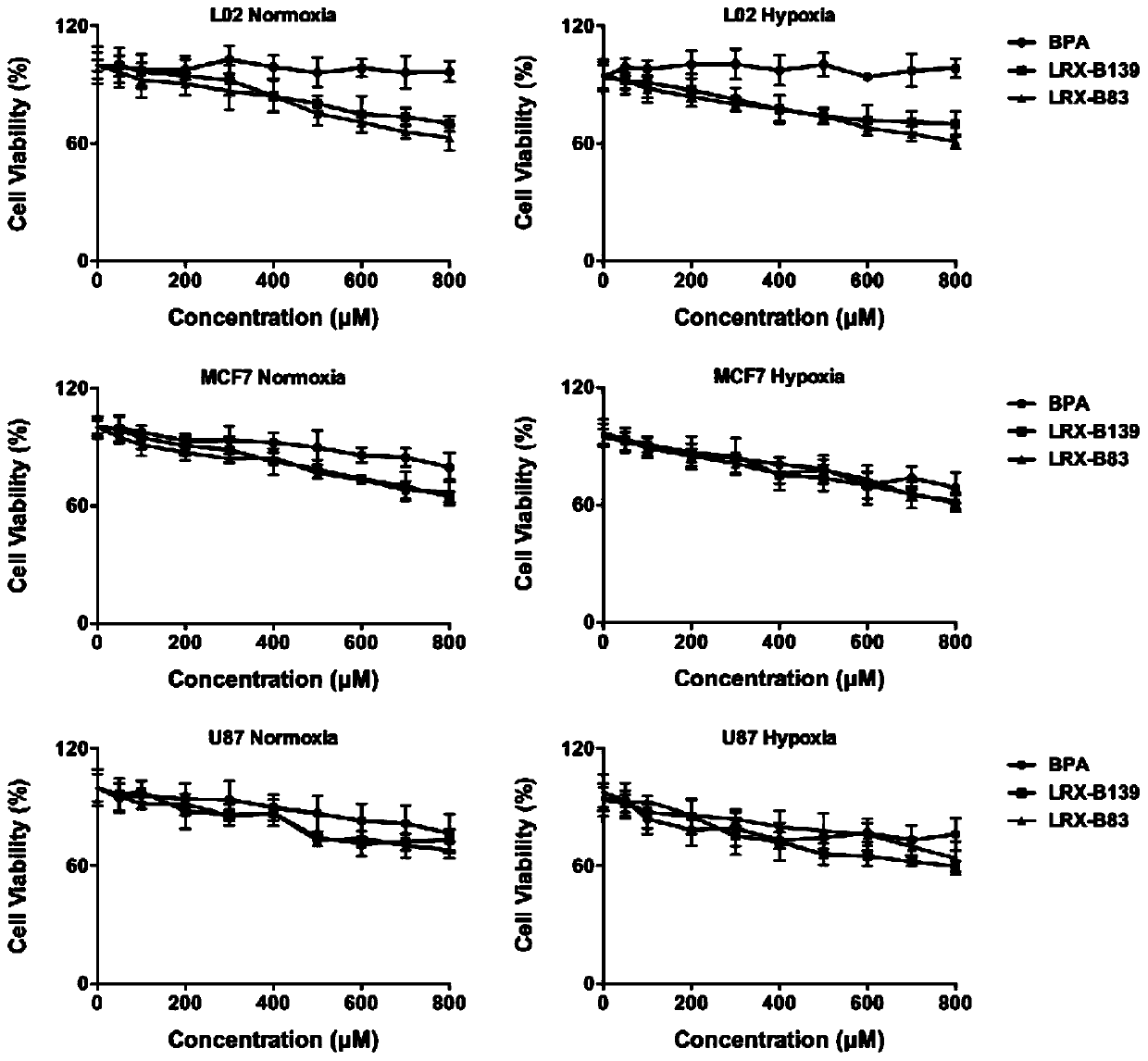
[0132] Experimental example: Cytotoxicity experiment
[0133] U87 cells, MCF-7 cells and L02 cells were cultured in DMEM incomplete high-glucose medium supplemented with 10% (v / v) fetal bovine serum, penicillin (80U / ml), streptomycin (0.08mg / ML) , placed at 37°C, 5% CO 2 cultured in a constant temperature incubator.
[0134] (1) U87 cells, MCF-7 cells and L02 cells in the logarithmic growth phase were treated with 0.25% trypsin digestion solution to make a concentration of 1×10 6 Add 100 μL of cell suspension to each well of a 96-well cell culture plate and incubate for 24 hours.
[0135] (2) Add concentration gradient probes after the cells adhere to the wall, design 5 replicate wells for each concentration in a 96-well plate, set a set of blank zero wells for each plate, and add cell suspension without adding drugs. Among them, the compound was prepared with medium to double the required concentration, and 100 μL was added to the corresponding wells respectively, and the ...
Embodiment
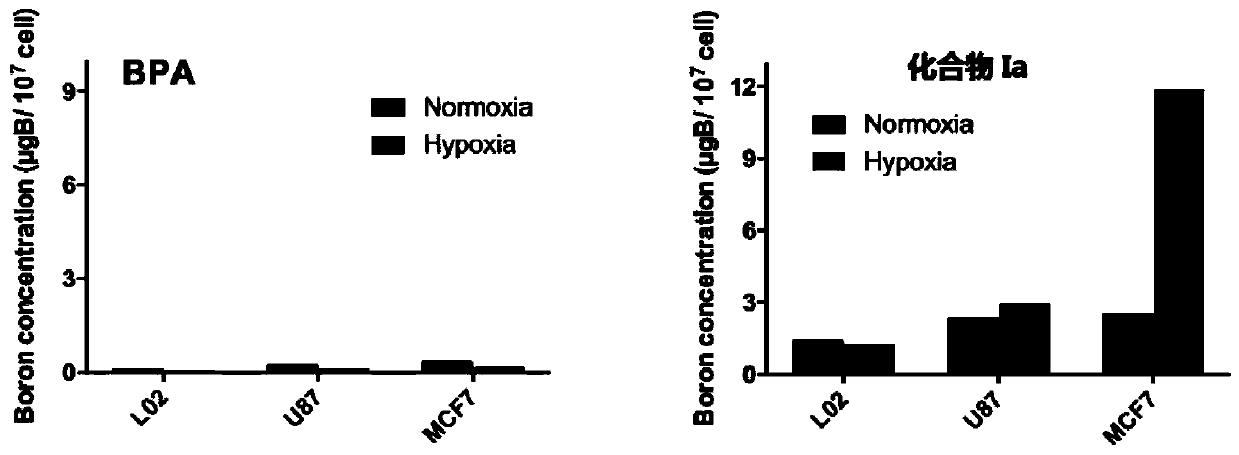
[0140] Example: Evaluation of boron uptake ability of boron-containing drugs in different cell lines
[0141] In order to investigate the enrichment ability of this type of boron drug in the hypoxic part of the tumor, each type of cell was divided into a normoxic group and a hypoxic group for the experiment. The normoxia group was placed in a cell incubator with an oxygen concentration of 20%, and the anoxic group was placed in a three-gas incubator with an oxygen concentration of 1%.
[0142] (1) Three cell lines, human normal liver cell L02, human breast cancer cell MCF-7, and human glioma cell U87, were taken out of the cryopreservation box and put into a 37°C water bath to quickly thaw. Take out the thawed liquid at 1000g, centrifuge for 5min, remove the supernatant, add 1ml of medium to the centrifuge tube and blow the cell pellet evenly, add the blown cell suspension into the cell culture dish, then add 7ml DMEM for culture base. Shake to make it evenly mixed, and then...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com