Synthesis method of repaglinide
A synthetic method and compound technology, applied in the field of drug synthesis, can solve problems such as poor stereoselectivity and low yield, and achieve the effects of good quality, high reaction conversion rate, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
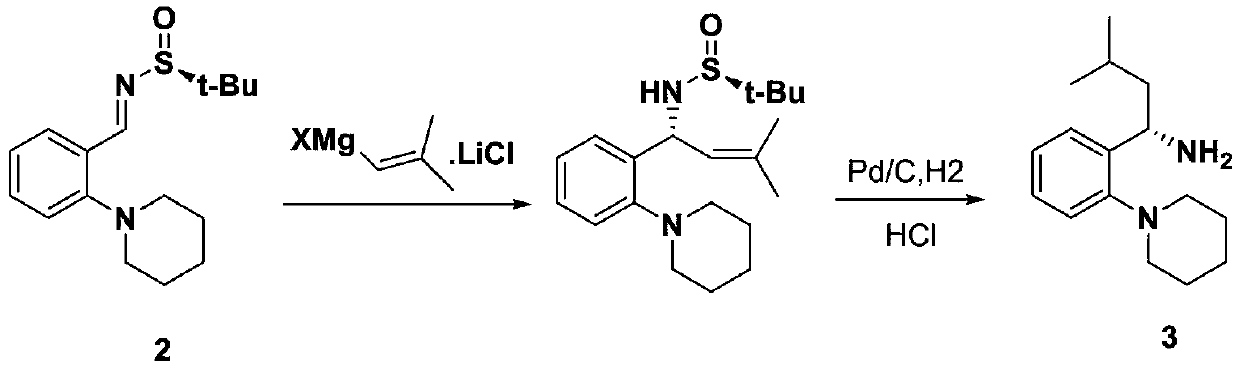
Examples
Embodiment 1
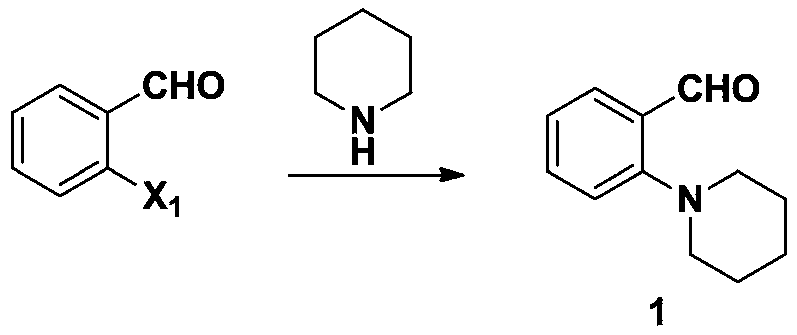
[0044] Synthesis of step a 2-piperidine-1-benzaldehyde (1)
[0045] 62.0g (0.5mol, 1.0eq) of o-fluorobenzaldehyde, 200g of N,N-dimethylformamide, 138.2g (1.0mol, 2.0eq) of potassium carbonate, and 63.9g (0.75mol, 1.5eq) of piperidine were reacted In the bottle, heated to reflux for 15 hours, the reaction of the raw materials controlled by TLC was complete, and the temperature was lowered. The reaction solution was poured into ice water, extracted with methyl tert-butyl ether, and the organic layers were combined and concentrated under reduced pressure to obtain 87.1 g of a yellow oil with a purity of 95.3% by HPLC. Yield 92.1%. 1 H (400MHz, CDCl 3):δ10.26(s,1H),7.75(dd,J=7.4Hz,J=1.8Hz,1H),7.46(ddd,J=8.3Hz,J=7.4Hz,J=1.8Hz,1H), 7.09-7.02(m,2H),3.01(t,J=5.4Hz,4H),1.77-1.73(m,4H),1.60-1.54(m,2H).MS(m / z)=190.1[M+ 1] +
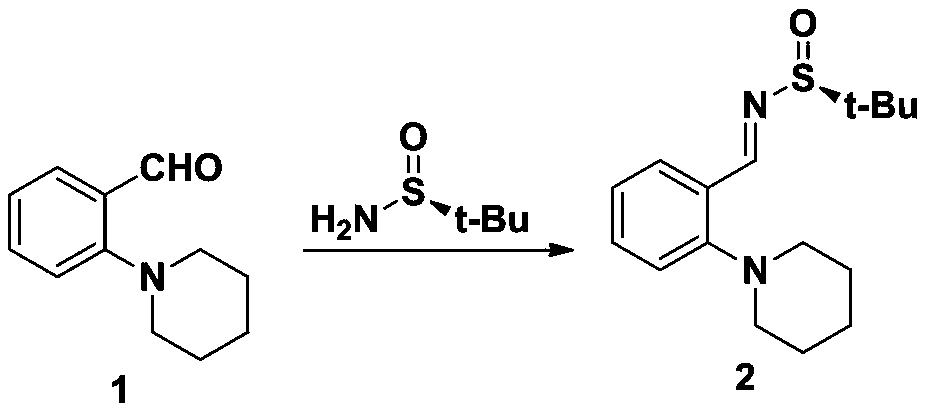
[0046] Synthesis of step b sulfinyl imide (2)
[0047] Compound 187.1g (0.46mol, 1.0eq), pyridine 4-methylbenzenesulfonate 3.5g (13.8mmol, 0.03eq), (R)-(+)-te...
Embodiment 2
[0056] Synthesis of step a 2-piperidine-1-benzaldehyde (1)
[0057] 70.3g (0.5mol, 1.0eq) of o-chlorobenzaldehyde, 210g of N,N-dimethylformamide, 488.7g of cesium carbonate (1.5mol, 3.0eq), 106.4g of piperidine (85.15, 1.25mol, 2.5eq) Put it in a reaction bottle, heat and reflux for 11 hours, the reaction of the raw material in TLC is complete, lower the temperature, pour the reaction solution into ice water, extract with methyl tert-butyl ether, combine the organic layers and concentrate under reduced pressure to obtain 89.8 g of yellow oil, HPLC purity 96.0 %, yield 94.9%, MS(m / z)=190.1[M+1] +
[0058] Synthesis of step b sulfinyl imide (2)
[0059] Compound 189.8g (0.47mol, 1.0eq), pyridine 4-methylbenzenesulfonate 1.2g (4.8mmol, 0.01eq), (R)-(+)-tert-butylsulfinyl 86.2g (0.71mol, 1.5 eq) and 450g of dichloromethane were placed in a reaction flask, heated to reflux for 10 hours, the remaining 8% of the raw material was controlled in HPLC, the temperature was lowered, 2g ...
Embodiment 3
[0068] Synthesis of step a 2-piperidine-1-benzaldehyde (1)
[0069] 92.5g (0.5mol, 1.0eq) of o-bromobenzaldehyde, 300g of N,N-dimethylsulfoxide, 276.4g (2.0mol, 4.0eq) of potassium carbonate, and 127.7g (1.5mol, 3.0eq) of piperidine In the bottle, heated to reflux for 15 hours, the reaction of the raw materials controlled by TLC was complete, and the temperature was lowered. The reaction solution was poured into ice water, extracted with methyl tert-butyl ether, and the organic layers were combined and concentrated under reduced pressure to obtain 90.5 g of a yellow oil with a purity of 96.1% by HPLC. Yield 95.6%. MS(m / z)=190.1[M+1] +
[0070] Synthesis of step b sulfinyl imide (2)
[0071] Compound 190.5g (0.478mol, 1.0eq), pyridine 4-methylbenzenesulfonate 6.0g (23.9mmol, 0.05eq), (R)-(+)-tert-butylsulfinyl 144.8g (121.20, 1.19mol , 2.5eq) and 450g of 1,4-dioxane were placed in a reaction flask, heated to reflux for 5 hours, and the reaction of raw materials in HPLC was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com