Polyether chain substituted alkyne-based (I) complex and preparation method and application thereof
A complex and fund technology, which is applied in the direction of gold organic compounds, active ingredients of heavy metal compounds, medical preparations containing active ingredients, etc., can solve the problems of limited efficacy and reduced stability in the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
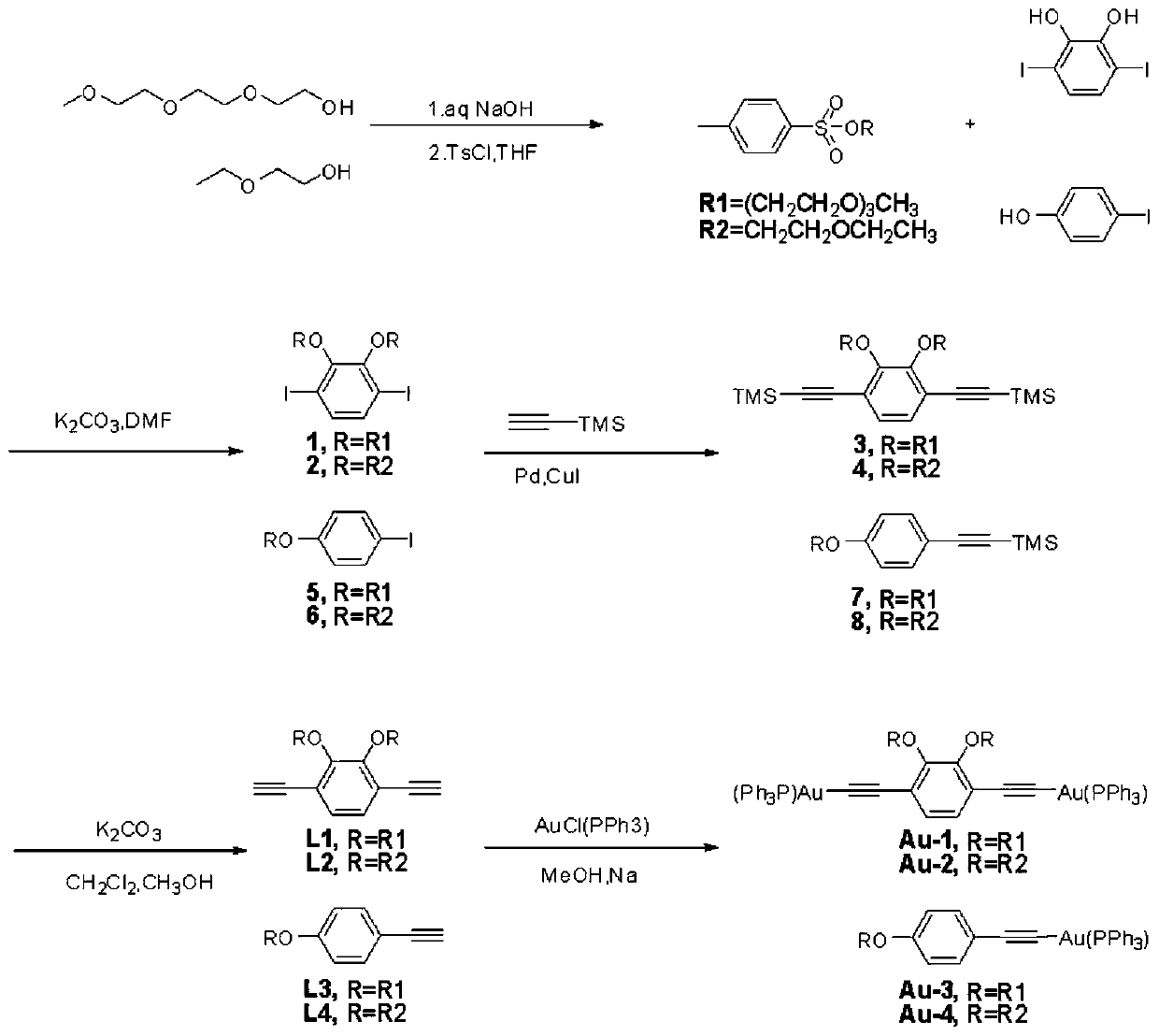
[0046] Embodiment 1: Alkyne base (I) complexes substituted by polyether chains, dinuclear alkyne base (I) complexes, the structural formula is
[0047] Wherein the R of Au-1 is (CH 2 CH 2 O) 3 CH 3 , R of Au-2 is CH 2 CH 2 OCH 2 CH 3 ; Mononuclear alkyne base (I) complexes, the structural formula is Wherein the R of Au-3 is (CH 2 CH 2 O) 3 CH 3 , the R of Au-4 is CH 2 CH 2 OCH 2 CH 3 .
Embodiment 2
[0048] Embodiment 2: as figure 1 Shown, the preparation method of the dinuclear alkyne base (I) complex that polyether chain replaces, concrete steps are as follows:
[0049] (1) Add 75mL NaOH aqueous solution to 150mL tetrahydrofuran (THF) at 0°C, then add 10mmol triethylene glycol monomethyl ether or 10mmol ethylene glycol ether, and add p-10mmol toluene in three equal portions within 30min Sulfonyl chloride (TsCl), reacted at room temperature under stirring conditions for 12h, quenched the reaction by adding water, stood to separate layers, extracted with dichloromethane to obtain the organic phase I, and the organic phase I was washed with water and brine successively and dried; The solvent is suspended and dried to obtain crude product A or crude product B. The crude product A or crude product B is eluted with ethyl acetate-petroleum ether through a chromatographic column, and the eluate is concentrated to obtain solid compound R1 or solid compound R2; wherein NaOH The m...
Embodiment 3
[0057] Embodiment 3: as figure 1 Shown, the preparation method of the mononuclear alkyne base (I) complex that polyether chain replaces, concrete steps are as follows:
[0058] (1) Add 75mL NaOH aqueous solution to 150mL tetrahydrofuran (THF) at 0°C, then add 10mmol triethylene glycol monomethyl ether or 10mmol ethylene glycol ether, and add p-10mmol toluene in three equal portions within 30min Sulfonyl chloride (TsCl), reacted at room temperature under stirring conditions for 12h, quenched the reaction by adding water, stood to separate layers, extracted with dichloromethane to obtain the organic phase I, and the organic phase I was washed with water and brine successively and dried; The solvent is suspended and dried to obtain crude product A or crude product B, and the crude product A or crude product B passes through a chromatography column, and is eluted with ethyl acetate-petroleum ether, and the eluate is concentrated to obtain solid compound R1 or solid compound R2; wh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com