Application of myricanol in preparing drug for resisting virulence of Gram-negative bacteria
A gram-negative bacteria, anti-bacterial technology, applied in the field of natural medicinal chemistry and pharmaceutical applications, can solve the problem of no relevant reports on the material basis of medicinal effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1. Separation of myricanol (myricanol)
[0027] Myricanol (myricanol) is separated from the dry bark of Chinese bayberry, and the separation method is as follows:
[0028] (1) Weigh 1.0 kg of dry bark of Bayberry, crushed and soaked in ethyl acetate for 3 times, extracted for two days each time, combined the extracts, and concentrated to dryness under reduced pressure at 40°C to obtain a crude extract;
[0029] (2) The crude extract is dissolved in water, and the total extract is sequentially extracted with petroleum ether, ethyl acetate and methanol, and the petroleum ether component, ethyl acetate component and methanol component are obtained after the solvent is recovered;
[0030] (3) The test results of anti-Salmonella T3SS activity show that the methanol component has better activity to inhibit the secretion of Salmonella virulence protein, so the methanol component is separated. First, it was chromatographed on Sephadex LH-20 gel column and eluted with methanol t...
Embodiment 2
[0031] Example 2. Structural identification of myricanol (myricanol)
[0032] Compound 1: ESI-MS measured the quasi-molecular ion peak of Compound 1 of Example 1 as m / z 359.2 [M+H] + ; White amorphous powder; 1 H, 13 C NMR and HMQC spectra show that the compound has 21 carbon atoms, including 12 sp 2 Hybrid olefinic carbon, 6 sp 3 Hybrid methine carbon, 1 sp 3 Hybrid methylene carbon atom and 2 methyl carbons. 1 H NMR data shows that the structure contains a tri-substituted benzene ring and a 5-substituted benzene ring, according to 1 H- 1 H COSY and HMQC determined the direct correlation between C-H; further through the long-range correlation between HMBC key protons and related carbons, it was determined that the structure of compound 1 is a cyclic diarylheptane compound myricanol (myricanol). The NMR data are as follows, 13 CNMR(400MHz, in acetone-d 6 ): δ125.0(C-1), 123.6(C-2), 147.6(C-3), 140.4(C-4), 149.8(C-5), 123.9(C-6), 26.3(C- 7), 26.5(C-8), 23.8(C-9), 40.4(C-10), 35.5(C...
Embodiment 3
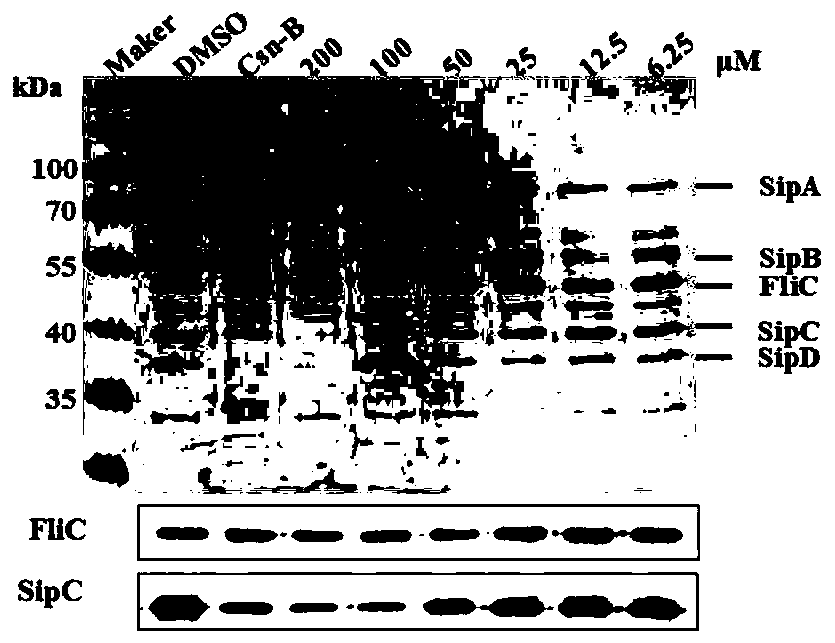
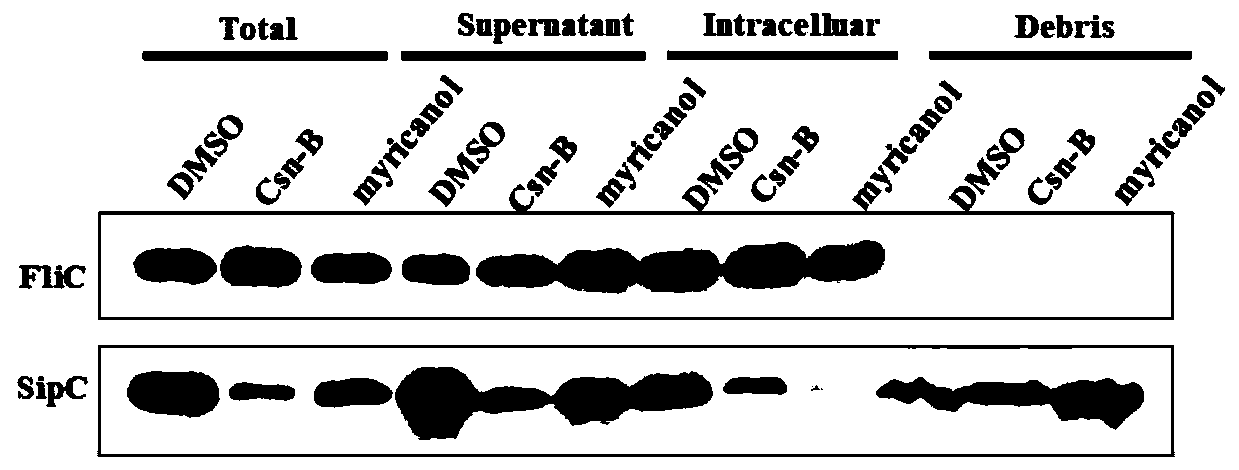
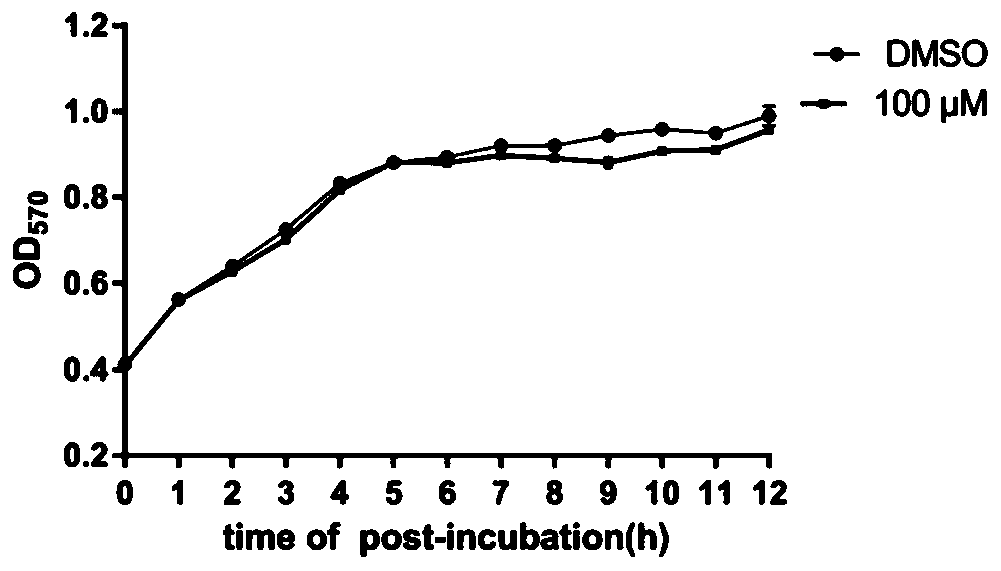
[0033] Example 3. Inhibition of myricanol on the secretion of SipA, B, C and D of Salmonella type III virulence protein in vitro
[0034] Test principle:
[0035] The type III secretion system is a very important virulence factor in the pathogenic process of gram-negative bacteria. It uses the type III secretion system to secrete virulence proteins to promote the adhesion and invasion of host cells, as well as the reproduction and spread of the host cells. . Therefore, blocking the secretion of virulence proteins can inhibit the invasion of host cells by Gram-negative pathogens. In this experiment, Salmonella typhimurium UK-1 (χ8956) was used as the research object. Through the temperature induction method, Salmonella typhimurium secreted virulence proteins into the culture medium through the type III secretion system, and detected by SDS-PAGE and Western Blot. To evaluate the inhibitory effect of myricanol on the secretion of virulence proteins.
[0036] Test materials:
[0037] S...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com