Method for preparing nano alpha-Fe2O3
A kind of -fe2o3, nanotechnology, applied in the direction of nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve the problems of uneven nucleation, high cost, complex process, etc., to expand the range of raw materials, reduce Production cost, effect of improving utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
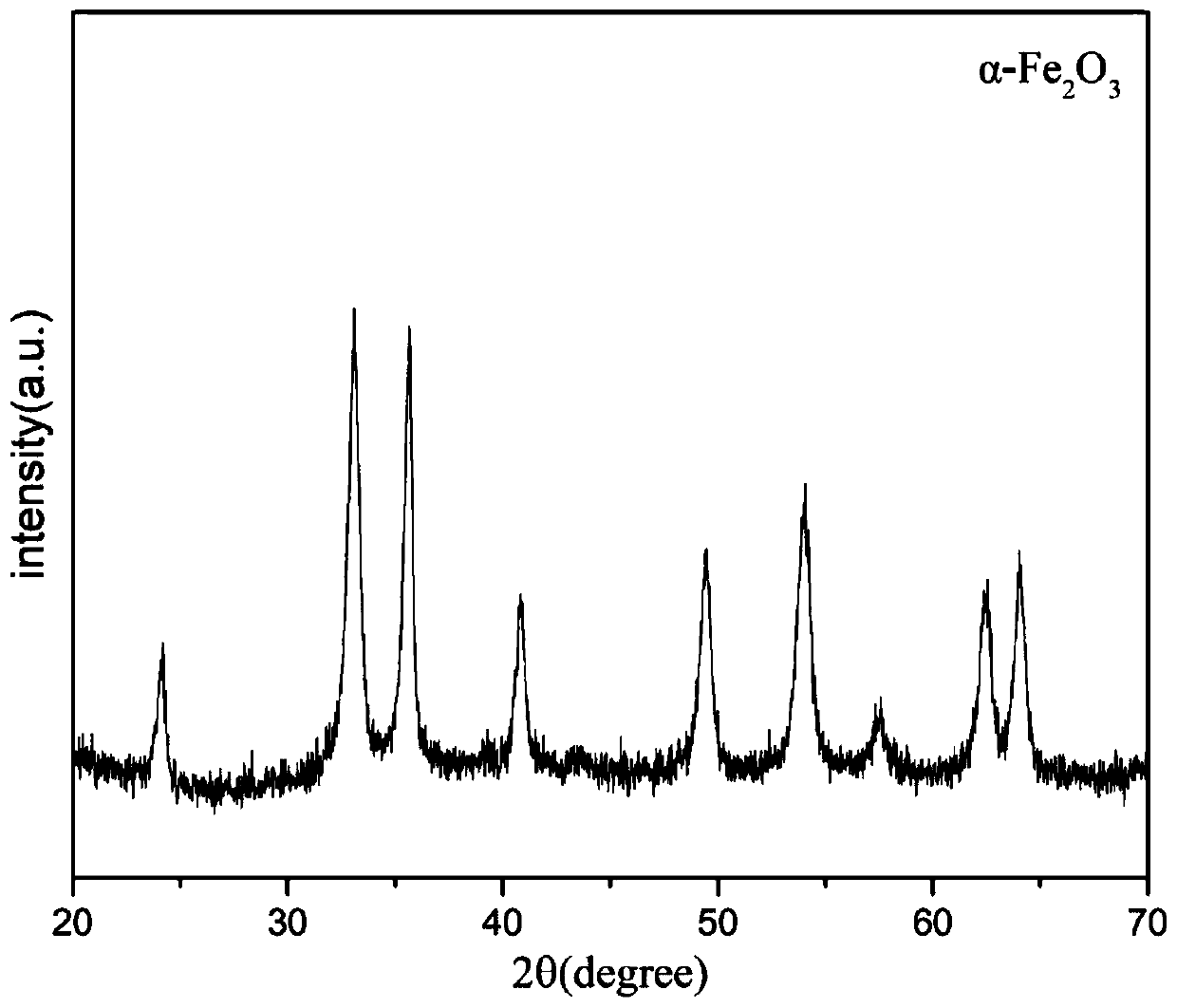
[0024] with FeCl 3 ·6H 2 O is raw material, get 1.35g FeCl 3 ·6H 2 O crystal plus 1.59g Na 2 CO 3 Mix and grind for 5 minutes, put the mixture into a sealed stainless steel reaction kettle and raise the temperature to 150°C for crystallization at a constant temperature for 54 hours. After the crystallization is completed, the filter cake is rapidly cooled, washed, and dried to obtain a crystallized product. Tested by X-ray diffraction (XRD), the product is α-Fe 2 o 3 , α-Fe 2 o 3 The crystal size is 15-20nm. Its XRD diagram and electron microscope diagram are shown in figure 1 and figure 2 .
Embodiment 2
[0026] Use iron mud as raw material, dissolve it with hydrochloric acid after high-temperature modification, heat and concentrate, cool and crystallize, and obtain ferric chloride crystals after drying, take 1.35g FeCl 3 ·6H 2 O crystal plus 1.59g Na 2 CO 3 Mix and grind for 5 minutes, put the mixture into a sealed stainless steel reaction kettle and raise the temperature to 150°C for crystallization at a constant temperature for 54 hours. After the crystallization is completed, the filter cake is rapidly cooled, washed, and dried to obtain a crystallized product. Tested by X-ray diffraction (XRD), the product is α-Fe 2 o 3 , α-Fe 2 o 3 The crystal size is 15-20nm.
Embodiment 3
[0028] Use converter ash as raw material, separate iron-containing substances through superconducting equipment, dissolve the iron-containing substances with hydrochloric acid after high-temperature modification, heat and concentrate, cool and crystallize, and obtain ferric chloride crystals after drying. Take 1.35g FeCl 3 ·6H 2 O crystal plus 1.59gNa 2 CO 3 Mix and grind for 5 minutes, put the mixture into a sealed stainless steel reaction kettle and raise the temperature to 150°C for crystallization at a constant temperature for 54 hours. After the crystallization is completed, the filter cake is rapidly cooled, washed, and dried to obtain a crystallized product. Tested by X-ray diffraction (XRD), the product is α-Fe 2 o 3 , α-Fe 2 o 3The crystal size is 15-20nm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com