Effectiveness determination marker in disease treatment by pd-1 signal inhibitor
A technology of PD-1 and signal inhibition, applied in the direction of disease diagnosis, allergic diseases, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
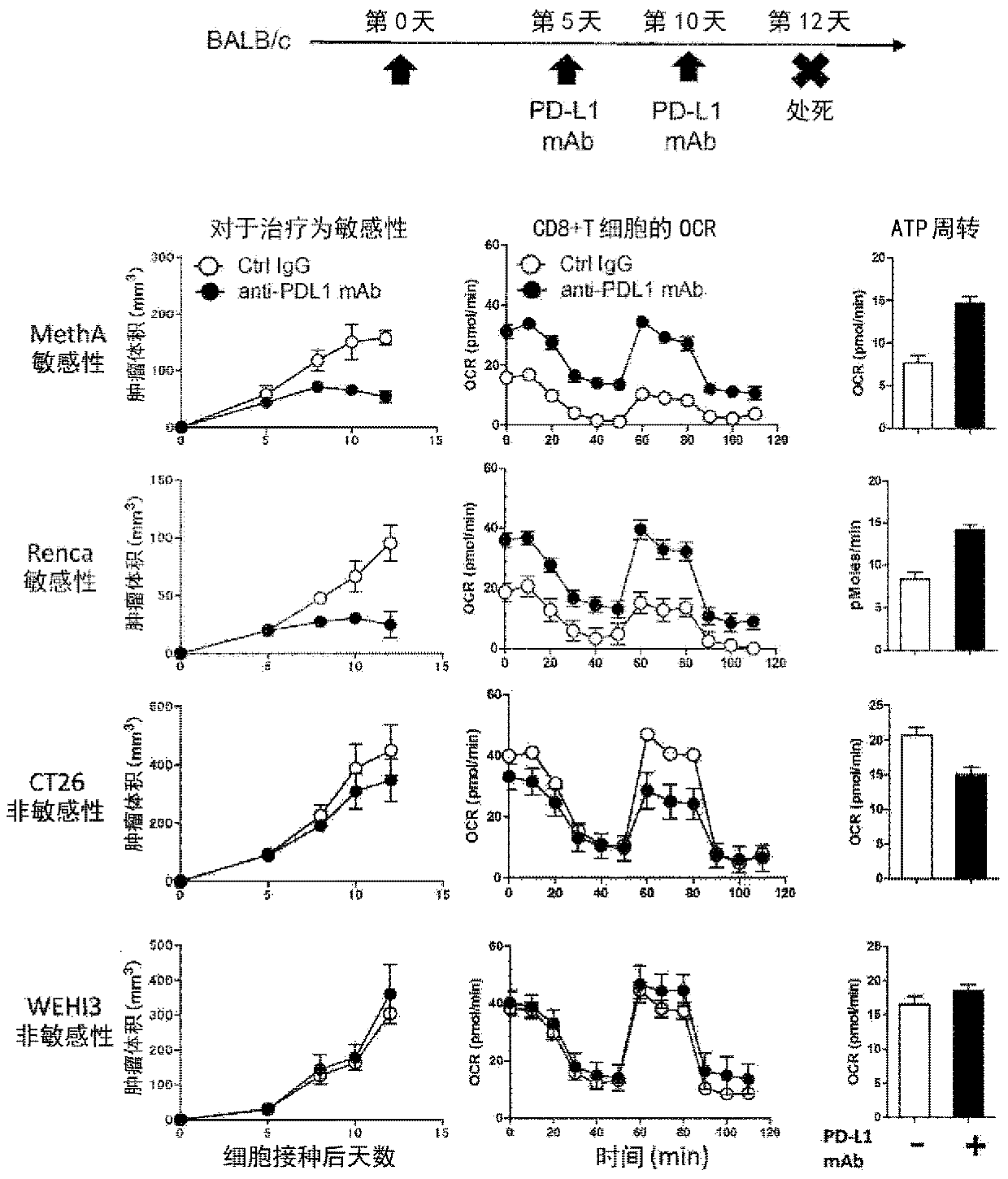
[0128] Meth A (Cell Resource Center for Biomedical Research) and RENCA (American Type Culture Collection), which are sensitive to PD-1 inhibitory antibody treatment, and non-sensitive CT26 (American Type Culture Collection) BALB / c mice (Charles River Laboratories Japan) were inoculated with WEHI (American Type Culture Collection), and PD-L1 antibody (1-111A, produced by our laboratory, 150ug / ml). On the second day from the last administration, CD8+ T cells in the accessory lymph nodes were isolated, and the oxygen consumption of mitochondria was measured using XF96 Extracellular Flux analyzer (Seahorse Biosciences). And based on it, ATP turnover is calculated. show the result in figure 1 .
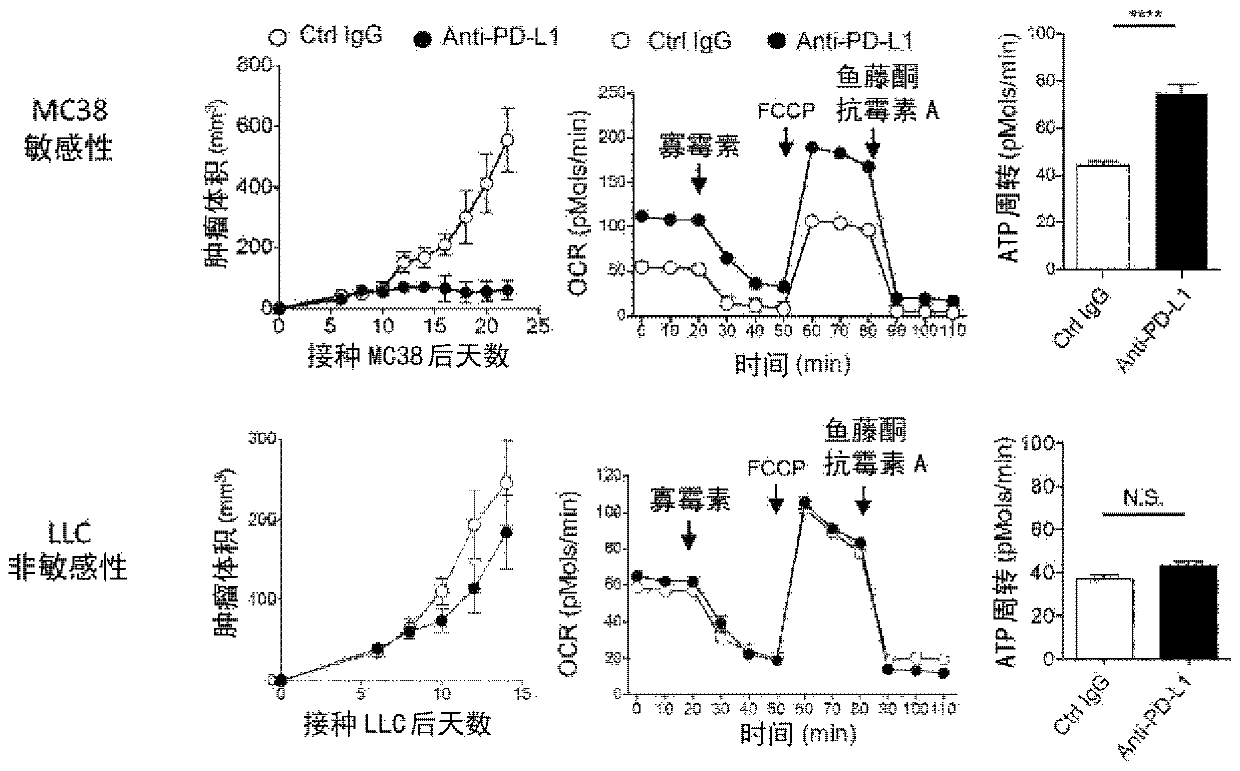
[0129] C57BL / 6N mice (Charles River Laboratories Japan) will be inoculated with MC38 (Dr. Jim Allison), non-sensitive LLC (American Type Culture Collection) sensitive to PD-1 inhibitory antibody treatment, 5 days later The PD-L1 antibody (1-111A, produced by our laboratory, 150ug / ml) ...
Embodiment 2
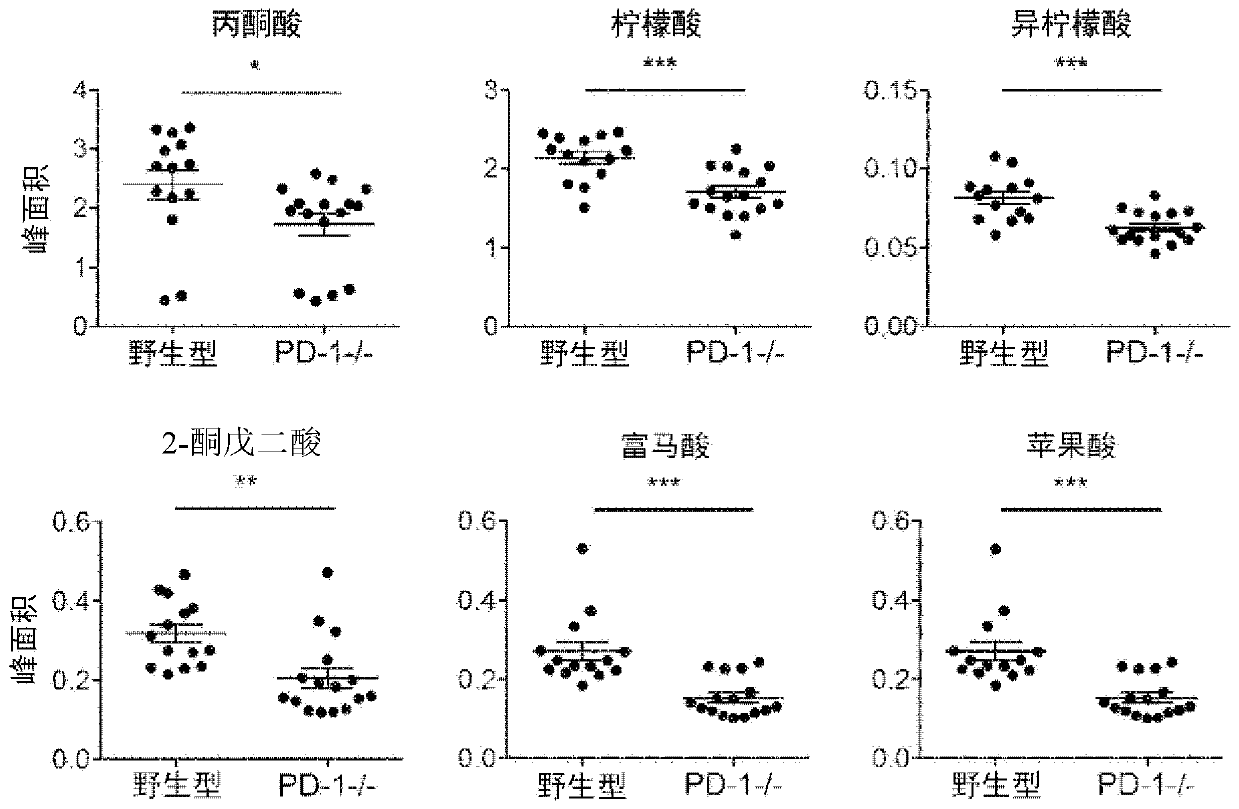
[0132] Extraction from wild-type C57BL / 6N mice (Charles River Laboratories Japan) and PD using a methanol / chloroform / water mixture - / - The serum recovered from mice (Immunity 11, 141-151 (1999).; Science 291, 319-322 (2001).; Nat. Med. 9, 1477-1483 (2003).) generated methoxylamine derivatives thing. After separation of metabolites using a methylpolysiloxane nonpolar column, the concentrations of TCA cycle-related metabolites were determined using mass spectrometry. In PD-1 - / - In mice, serum TCA cycle-related metabolites tended to decrease. show the result in image 3 .
[0133] C57BL / 6N mice were administered with PD-L1 antibody (1-111A, produced by our laboratory, 200ug) or control IgG (Bio X Cell) three times a day, and the serum levels related to the TCA cycle were determined by GC-MS. The concentration of metabolites. show the result in Figure 4 . When PD-L1 antibody is administered, metabolites related to TCA cycle in serum tend to decrease. This is considered ...
Embodiment 3
[0136] Mitochondrial activation is associated with cellular activation. In the activation of cells, mTOR signaling plays an important function. Mitochondria are also known to be activated for the mTOR pathway, and T-bet, which is important for Th1-type immunity, is also known to be activated.
[0137] Therefore, using the CD8+ T cells obtained in Example 1, the T-bet activity before and after treatment was compared.
[0138] The accessory lymph node cells used in Example 1 were stained with anti-CD8 antibody (BioLegend) and anti-T-bet antibody (BioLegend), and anti-EOMES antibody (eBioscience), and CD8+T cells were gated and compared in PD-L1 antibody The expression of T-bet and EOMES before and after administration. show the result in Figure 5 .
[0139] It is known that the expression of T-bet increases after antibody administration in the treatment of highly sensitive cancer. On the other hand, EOMES, which performs a different function from T-bet, increased regardles...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com