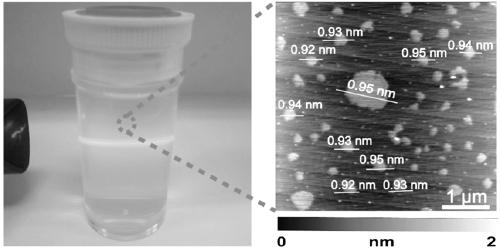
Preparation method of acid type niobium phosphate oxygen monoatomic layer sheet
A technology of niobium monophosphate and niobium phosphate precursor, which is applied in chemical instruments and methods, phosphorus compounds, inorganic chemistry, etc., to achieve the effects of high peeling yield, low cost and high efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Weigh 0.5g of commercial-grade niobium oxalate powder, transfer it to a 250mL three-necked flask that has been pre-cleaned and dried, and add 80mL of deionized water and 5mL of commercial-grade phosphoric acid in turn; The three-necked bottle was transferred and fixed on the oil bath reaction system equipped with a condensing reflux device, and the reaction solution was continuously stirred until a transparent and clear solution was formed, then the reaction temperature was adjusted to 120° C. for 15 hours of reaction. After the reaction, when the system was cooled to room temperature, the white precipitated product at the bottom of the bottle was transferred to a Buchner funnel covered with two layers of filter paper and completely sealed on the suction filter bottle, and the white product was extracted five times with deionized water. Filtration and washing operations, and the fully washed product was transferred to a blast drying oven and dried at 60°C for 8 hours to ...
Embodiment 2
[0030] Weigh 0.5g of commercial-grade niobium oxalate powder, transfer it to a 250mL three-necked flask that has been pre-cleaned and dried, and add 80mL of deionized water and 5mL of commercial-grade phosphoric acid in turn; The three-necked bottle was transferred and fixed on the oil bath reaction system equipped with a condensing reflux device, and the reaction solution was continuously stirred until a transparent and clear solution was formed, then the reaction temperature was adjusted to 120° C. for 15 hours of reaction. After the reaction, when the system was cooled to room temperature, the white precipitated product at the bottom of the bottle was transferred to a Buchner funnel covered with two layers of filter paper and completely sealed on the suction filter bottle, and the white product was extracted five times with deionized water. Filtration and washing operations, and the fully washed product was transferred to a blast drying oven and dried at 60°C for 8 hours to ...
Embodiment 3
[0032]Weigh 0.5g of commercial-grade niobium oxalate powder, transfer it to a 250mL three-necked flask that has been pre-cleaned and dried, and add 80mL of deionized water and 5mL of commercial-grade phosphoric acid in turn; The three-necked bottle was transferred and fixed on the oil bath reaction system equipped with a condensing reflux device, and the reaction solution was continuously stirred until a transparent and clear solution was formed, then the reaction temperature was adjusted to 120° C. for 15 hours of reaction. After the reaction, when the system was cooled to room temperature, the white precipitated product at the bottom of the bottle was transferred to a Buchner funnel covered with two layers of filter paper and completely sealed on the suction filter bottle, and the white product was extracted five times with deionized water. Filtration and washing operations, and the fully washed product was transferred to a blast drying oven and dried at 60°C for 8 hours to o...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com