P-alkoxyphenyl fullerene as well as preparation method and application thereof
A technology of p-alkoxyphenyl and alkoxyphenyl, which is applied in the field of p-alkoxyphenyl fullerene and its preparation, can solve the problems of poor stability and adaptability of stabilizers, and achieve stable performance, Improve comprehensive performance and prolong storage period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
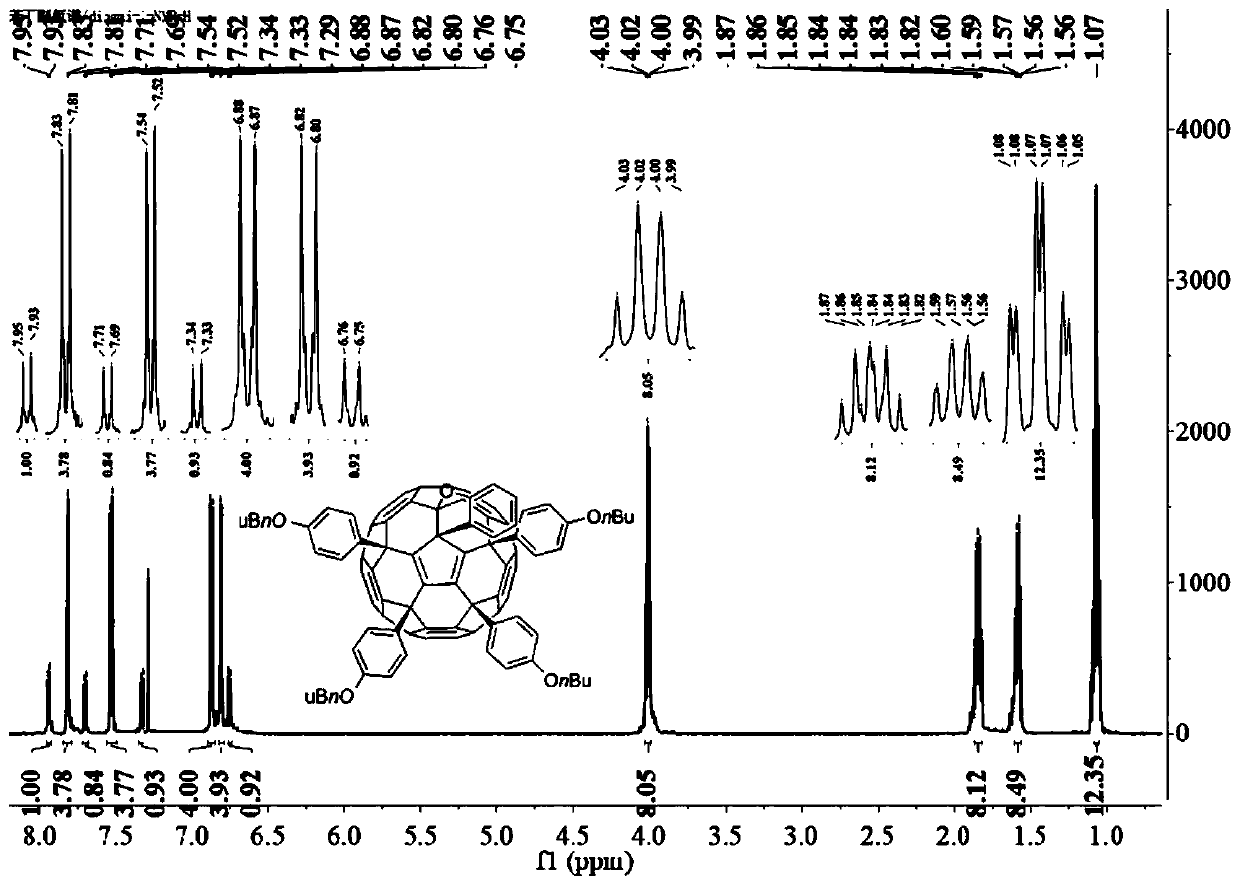
Examples
Embodiment 1
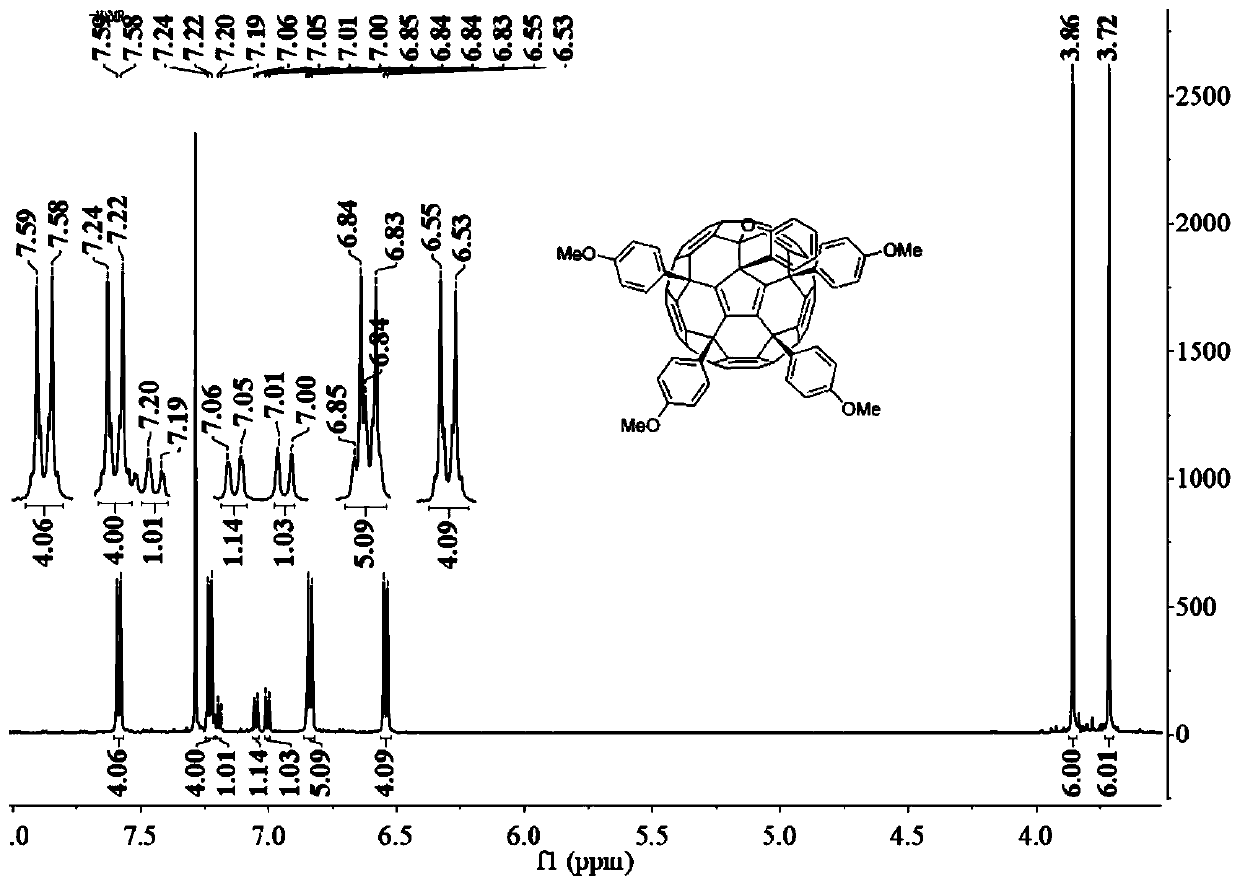
[0026] The preparation method of p-methoxyphenyl fullerene (a):
[0027] At a room temperature of 25° C., 0.05 mmol of hexachlorofullerene was dissolved in 20 mL of nitrobenzene, and the mixed solution was stirred and dissolved under nitrogen protection for 2 hours. After being completely dissolved, 6 mmol of methylphenyl ether and 1 mmol of anhydrous ferric chloride were added to the mixed solution, and the mixture was reacted at a constant temperature of 25°C. The reaction was stirred under nitrogen protection until thin layer chromatography showed that the reactant was completely consumed, and the reaction solution changed from orange to dark brown. After the reaction, the excess solvent was removed by rotary evaporation to obtain a reddish-brown crude product. The crude product was dissolved in a small amount of dichloromethane, and the orange-red product was obtained by separation by silica gel column chromatography, and the eluent was dichloromethane / carbon disulfide (1...
Embodiment 2
[0029] The preparation method of p-methoxyphenyl fullerene (a):
[0030] At a room temperature of 25° C., 0.05 mmol of hexachlorofullerene was dissolved in 100 mL of dichloromethane, and the mixed solution was stirred and dissolved under nitrogen protection for 2 hours. After being completely dissolved, 6 mmol of methylphenyl ether and 1 mmol of anhydrous aluminum trichloride were added to the mixed solution, and the mixture was reacted at a constant temperature of 25°C. The reaction was stirred under nitrogen protection until thin layer chromatography showed that the reactant was completely consumed, and the reaction solution changed from orange to dark brown. After the reaction, the excess solvent was removed by rotary evaporation to obtain a reddish-brown crude product. The crude product was dissolved in a small amount of dichloromethane, and the orange-red product was obtained by separation by silica gel column chromatography, and the eluent was dichloromethane / carbon dis...
Embodiment 3
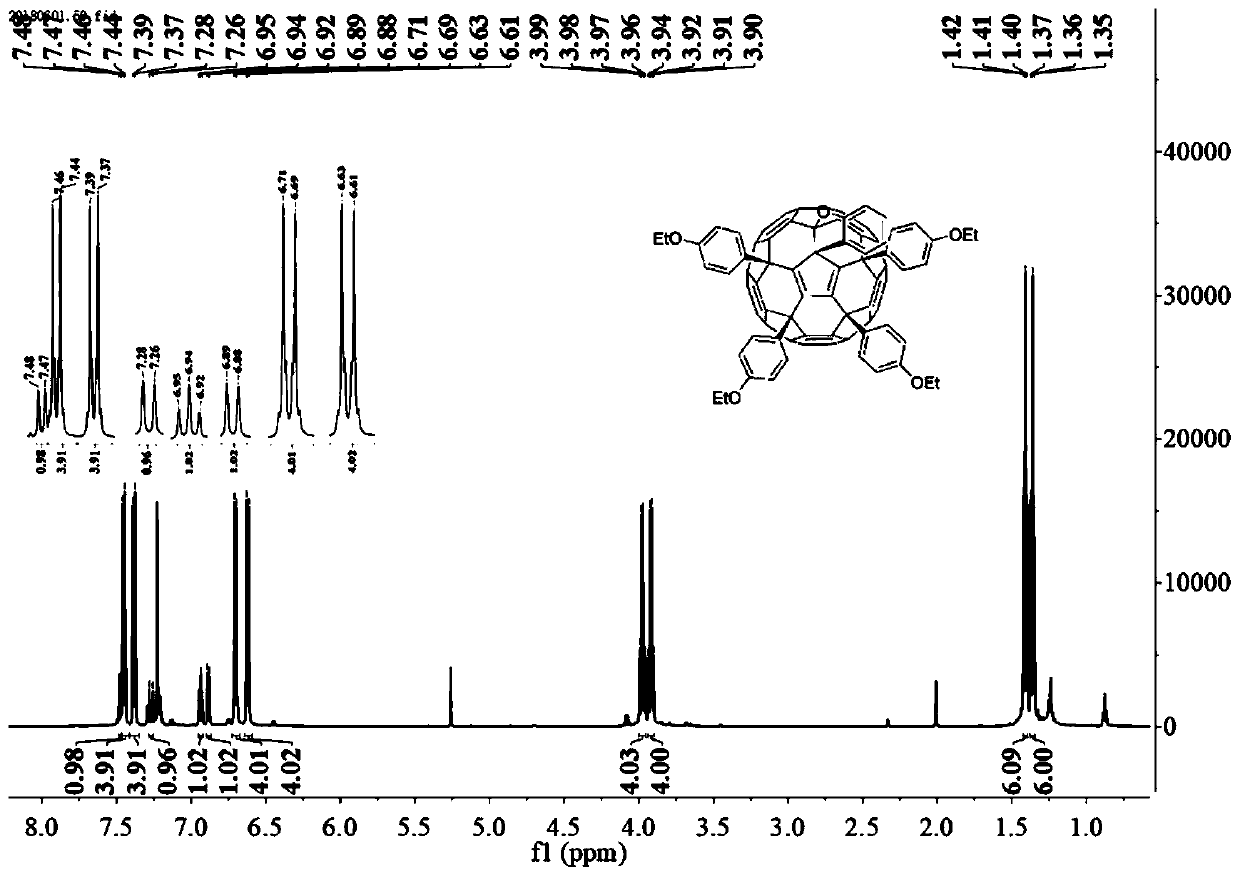
[0032] The preparation method of p-ethoxyphenyl fullerene (b):
[0033] At a room temperature of 25° C., 0.05 mmol of hexachlorofullerene was dissolved in 100 mL of dichloromethane, and the mixed solution was stirred and dissolved under nitrogen protection for 2 hours. After the solution was completely dissolved, 6 mmol of ethyl phenyl ether and 1 mmol of anhydrous aluminum trichloride were added to the mixed solution, and the temperature of the water bath was raised to 50°C. The reaction was stirred under nitrogen protection until thin layer chromatography showed that the reactant was completely consumed, and the reaction solution changed from orange to dark brown. After the reaction, the excess solvent was removed by rotary evaporation to obtain a reddish-brown crude product. The crude product was dissolved in a small amount of dichloromethane, and the orange-red product was obtained by separation by silica gel column chromatography, and the eluent was dichloromethane / carbo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com