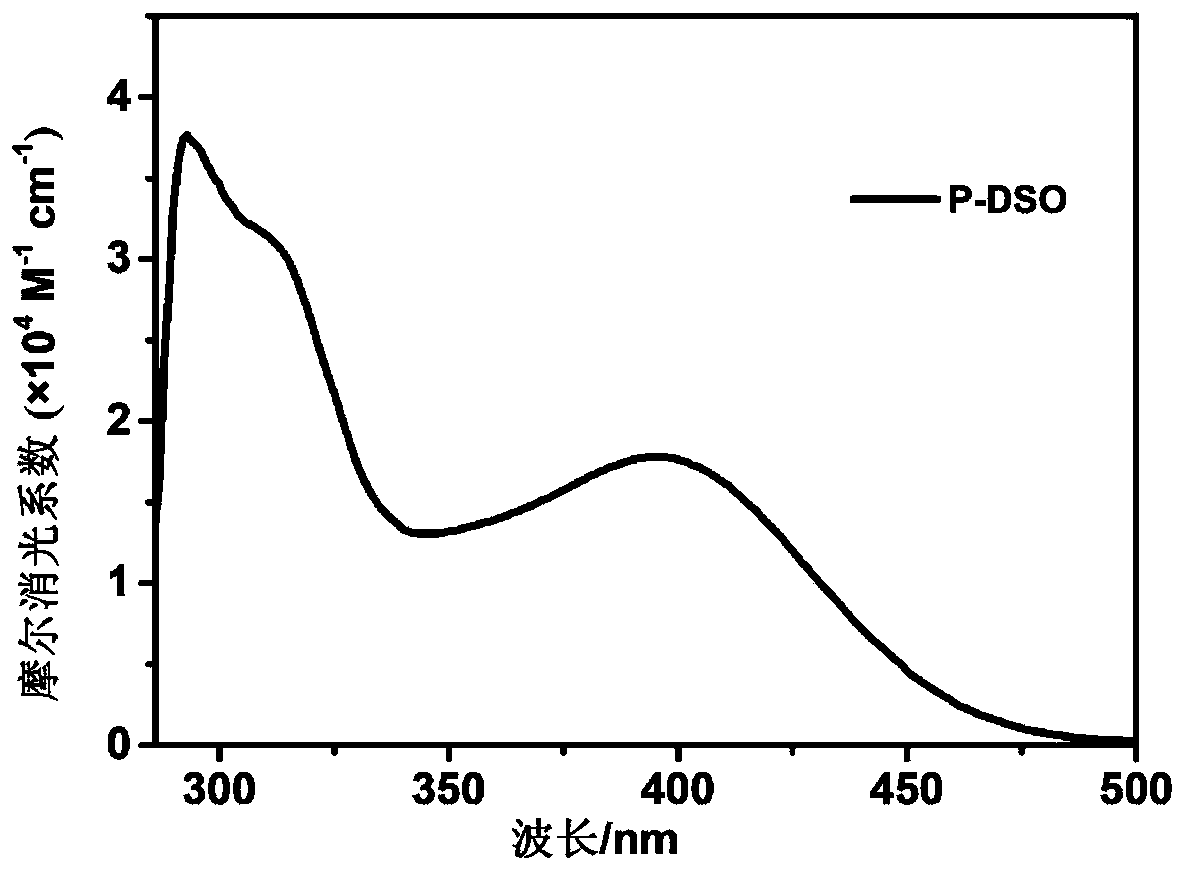
Diphenyl sulfone-based A-pi-D-pi-A type sensitizer as well as preparation method and application thereof
A technology of diphenyl sulfone and sensitizer, applied in the field of A-π-D-π-A type sensitizer and its preparation, can solve the problem of mismatch of absorption wavelength of photoinitiator, achieve fast photobleaching, excellent Effects of Intramolecular Charge Transfer Capability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
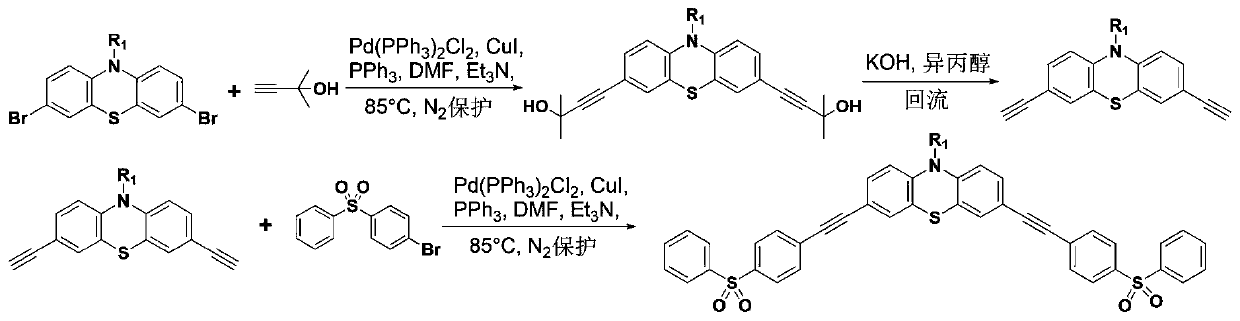
[0046] like figure 1 Shown, N-dodecyl-3,7-dibromophenothiazine (17.86g, 40mmol), CuI (0.7618g, 4mmol), PPh 3 (1.0492g, 4mmol), Pd(PPh 3 ) 2 Cl 2 (0.2808g, 0.4mmol) were mixed, 100mL DMF and 50mL triethylamine were added, stirred magnetically under nitrogen atmosphere, heated to 60°C and kept for 1h, then heated to 85°C. Then 2-methyl-3-butyn-2-ol was added dropwise, and the first product was obtained after reacting for 6 hours. During the reaction, the progress of the reaction was monitored by thin-layer chromatography. After the first product was cooled to room temperature, it was extracted with dichloromethane, and the organic phases were combined, washed with water and saturated brine, dried over anhydrous sodium sulfate, purified through a silica gel column with ethyl acetate / petroleum ether as the eluent, and used Dissolve 150mL of isopropanol, add 10 molar equivalents of KOH, stir and heat to reflux for 4 hours under nitrogen atmosphere, after cooling to room tempera...
Embodiment 2
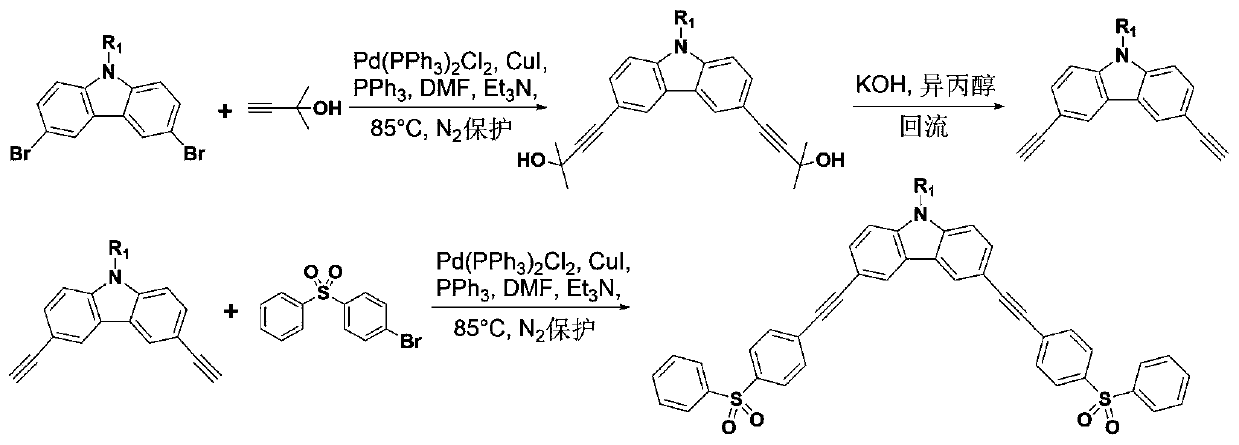
[0056] like image 3 As shown, the difference from Example 1 is that the reaction compound of this example is N-dodecyl-3,6-dibromocarbazole.
[0057] The intermediate is N-dodecyl-3,6-diethynylcarbazole.
[0058] H NMR spectrum characterization data of the intermediate:
[0059] 1 H NMR (400MHz, DMSO-d 6 )δ (ppm): 8.40 (d, J = 1.4Hz, 2H), 7.64 (s, 1H), 7.62 (s, 1H), 7.56 (dd, J = 8.4, 1.6Hz, 2H), 4.40 (t, J=7.0Hz, 2H), 1.73(q, J=7.1Hz, 2H), 1.27-1.12(m, 18H), 0.84(t, J=6.8Hz, 3H).
[0060] Carbon NMR spectrum characterization data of the intermediate:
[0061] 13 C NMR (DMSO-d 6 )δ (ppm): 140.19, 129.68, 124.53, 121.56, 112.24, 109.93, 84.66, 78.69, 42.44, 31.25, 28.91, 28.81, 28.79, 28.63, 28.37, 26.27, 22.05, 13.91%. Yield: 7
[0062] The final product is N-dodecyl-3,6-di(4-phenylsulfonylphenylethynyl)carbazole (C-DSO).
[0063] C-DSO H NMR spectrum characterization data:
[0064] 1 H NMR (400MHz, DMSO-d 6 )δ8.53(s,2H),8.12-7.90(m,8H),7.86-7.57(m,14H),4.43(t,J=7...
Embodiment 3
[0069] like Figure 5 As shown, the difference from Example 1 is that the reaction compound of this example is 4,4'-dibromotriphenylamine.
[0070] The intermediate is 4,4'-diethynyltriphenylamine.
[0071] H NMR spectrum characterization data of the intermediate:
[0072] 1 H NMR (400MHz, DMSO-d 6 )δ7.42-7.33(m,6H),7.20-7.14(m,1H), 7.11-7.06(m,2H),6.99-6.91(m,4H),4.09(s,2H).
[0073] Carbon NMR spectrum characterization data of the intermediate:
[0074] 13 C NMR (400MHz, d 6-DMSO) δ147.00, 145.88, 133.11, 132.99, 132.69, 129.89, 125.55, 124.68, 123.30, 122.83, 115.58, 83.39, 80.03. Yield: 62%.
[0075] The final product is 4,4'-bis(4-benzenesulfonylphenylethynyl)triphenylamine (T-DSO).
[0076] T-DSO H NMR spectrum characterization data:
[0077] 1H NMR (400MHz, Chloroform-d) δ (ppm): 7.99-7.92 (m, 4H), 7.92-7.86 (m, 4H), 7.59 (dd, J = 8.3, 1.7Hz, 5H), 7.57-7.47 ( m,5H),7.44-7.36(m,4H),7.34-7.28(m,2H),7.16-7.10(m,3H),7.09-7.00(m,4H).
[0078] Carbon spectrum chara...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com