Novel genetic engineering subunit vaccine for chicken mycoplasma synoviae
A technology of Mycoplasma synovialis and amino acids, which is applied in genetic engineering, vaccines, veterinary vaccines, etc., can solve the problems of difficult mycoplasma culture, low antigen content, weak autoimmune protection, etc., and achieves good immunogenicity and immunogenicity. The effect of strong performance and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0113] Example 1 Construction and Identification of Transfer Vector pF-MSPA
[0114] 1. MSPA gene amplification and purification The codon-optimized MSPA gene (SEQ ID NO: 1) was synthesized in Nanjing GenScript Company and cloned into the pUC17 vector to obtain the pUC-MSPA plasmid vector. The pUC-MSPA plasmid was used as a template, and MSPA-F and MSPA-R were used as upstream and downstream primers for PCR amplification (the gene sequences of MSPA-F and MSPA-R are shown in SEQ ID NO.5 and 6). For the amplification system, see Table 1.
[0115] Table 1 MSPA gene amplification system
[0116]
[0117] The reaction conditions were: 95°C pre-denaturation for 5 minutes; 94°C denaturation for 45 seconds, 54°C annealing for 45 seconds, 72°C extension for 1 minute, 35 cycles; 72°C extension for 10 minutes.
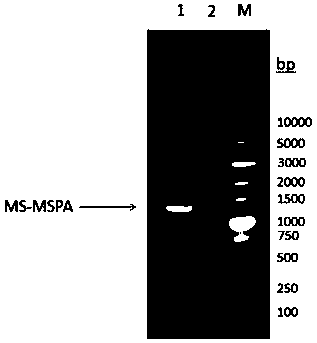
[0118] Perform gel electrophoresis on the PCR product to verify the size of the target gene, such as figure 1 As shown, the target band appeared at the position of 1.3kbp, ...
Embodiment 2
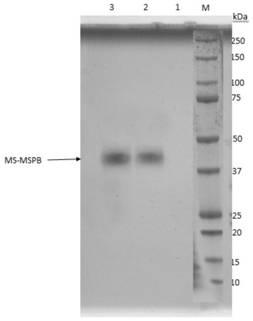
[0130] Example 2 Construction and Identification of Transfer Vector pF-MSPB
[0131] 1. MSPB gene amplification and purification The codon-optimized MSPB gene (SEQ ID NO: 3) was synthesized in Nanjing GenScript and cloned into the pUC17 vector to obtain the pUC-MSPB plasmid vector. The pUC-MSPB plasmid was used as a template, and MSPB-F and MSPB-R were used as upstream and downstream primers for PCR amplification (the gene sequences of MSPB-F and MSPB-R are shown in SEQ ID NO.7 and 8), and the amplification system is shown in table 5.
[0132] Table 5 MSPB gene amplification system
[0133]
[0134] The reaction conditions were: 95°C pre-denaturation for 5 minutes; 94°C denaturation for 45 seconds, 54°C annealing for 45 seconds, 72°C extension for 1 minute, 35 cycles; 72°C extension for 10 minutes.
[0135] Perform gel electrophoresis on the PCR product to verify the size of the target gene, such as image 3 As shown, the target band appears at the position near 1.0kbp, ...
Embodiment 3
[0147] Example 3 Construction of recombinant baculovirus genome Bac-MSPA and Bac-MSPB
[0148] 1. Transformation of DH10Bac bacteria Take 1 μl of pF-MSPA plasmid in Example 1 and 1 μl of pF-MSPB plasmid in Example 2 and add it to 100 μl of DH10Bac competent cells, mix evenly, ice-bath for 30 minutes, and heat shock in 42°C water bath for 90 seconds. Cool on ice for another 2 minutes, add 900 μl LB liquid medium without Amp, and incubate at 37° C. for 5 hours. After 100 μl of bacterial solution was diluted 81 times, 100 μl of diluted bacterial solution was spread on LB solid medium containing gentamicin, kanamycin, tetracycline, X-gal and IPTG, and cultured at 37°C for 48 hours.
[0149] 2. Select single clones and use inoculation needles to pick up large white colonies, then streak on LB solid medium containing gentamicin, kanamycin, tetracycline, X-gal and IPTG, and culture at 37°C for 48 hours , and then pick a single colony to inoculate the LB liquid medium containing gent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com