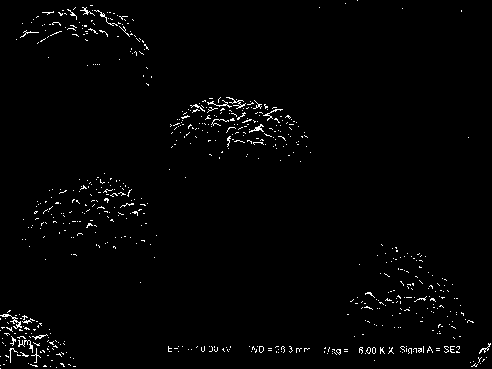
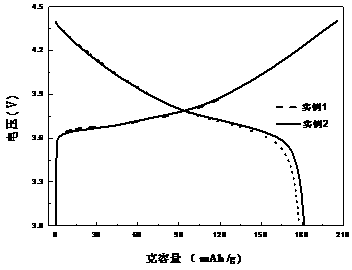
High-nickel lithium ion positive electrode material and preparation method thereof
A cathode material, high nickel lithium technology, applied in the direction of positive electrode, battery electrode, active material electrode, etc., can solve the problems of deterioration of discharge specific capacity, low first charge and discharge efficiency, and reduction of battery specific capacity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The first step is the preparation of high-nickel cathode material precursor:
[0035] a. Solution preparation: select nickel sulfate, cobalt sulfate, manganese sulfate, and magnesium nitrate, and prepare a 0.5mol / L metal salt solution according to the metal ion ratio of Ni:Co:Mn:Al:Mg=0.6:0.2:0.12:0.04 A1, including core material salt solution (Ni:Co=0.8:0.2) and shell material solution (Ni:Co:Al:Mg=0.4:0.2:0.24:0.08:0.08), and the volume ratio of core material to shell material is 5 : 5; The NaOH solution of 4mol / L is uniformly mixed with the ammoniacal liquor of 25% concentration by volume ratio 9:1, is mixed with mixed alkali solution B;
[0036] b. The preparation of the reaction kettle bottom liquid and the control of the reaction kettle conditions: inject deionized water into the reaction kettle, and adjust the pH value of the bottom liquid to 8 with ammonia water, control the temperature in the reaction kettle to 40°C, and the speed to 300rpm. Nitrogen, and thro...
example 2
[0053] The first step is the preparation of high-nickel cathode material precursor:
[0054] a, solution preparation: select nickel sulfate, cobalt sulfate, manganese sulfate, magnesium oxalate, be Ni:Co:Mn:Mg=0.9:0.05:0.03:0.02 according to metal ion ratio and be prepared into the metal salt solution A1 of 3mol / L, wherein , Prepare 3mol / L high nickel metal core material salt solution Ni:Co=0.95:0.05, 3mol / L first shell material Ni:Co:Mn:Mg=0.8:0.1:0.06:0.04, and second shell material Ni:Co:Mn:Mg=1 / 3: 1 / 3: 1 / 5:2 / 15. Wherein, the solution volume ratio of the core material and the two shell materials is 4 / 5:2 / 15:1 / 15, and the NaOH solution of 8mol / L and the disodium edetate solution of 15% concentration are mixed according to the volume ratio Mix 9:1 evenly to prepare mixed alkali solution B;
[0055] b. Preparation of reaction kettle bottom liquid and control of reaction kettle conditions: Inject deionized water into the reaction kettle, and adjust the pH value of the bottom ...
example 3
[0062] The first step is the preparation of high-nickel cathode material precursor:
[0063] a. Solution preparation: select nickel sulfate, cobalt sulfate, manganese sulfate, and sodium metaaluminate, and prepare 4mol / L metal salt solution A1 according to the metal ion ratio of Ni:Co:Mn:Al=0.855:0.1:0.03:0.02 , wherein the preparation of 4mol / L high nickel metal salt solution (Ni:Co=0.9:0.1) and 4mol / L high manganese metal salt solution (Ni:Co:Mn:Al=1 / 3:1 / 9:1 / 3:2 / 9), in which the volume ratio of high nickel solution and high manganese solution is 9:1, 10mol / L NaOH solution and 10% ammonia solution are uniformly mixed at a volume ratio of 6:4 to prepare a mixed alkali Solution B;
[0064] b. Preparation of reaction kettle bottom liquid and control of reaction kettle conditions: Inject deionized water into the reaction kettle, and adjust the pH value of the bottom liquid to 11 with ammonia water, control the temperature in the reaction kettle to 40°C, and the speed to 800rpm,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com