Aqueous electrolyte and aqueous metal ion battery
A water-based electrolyte and electrolyte technology, applied in aqueous electrolytes, secondary batteries, circuits, etc., can solve the problems of capacity retention or low Coulombic efficiency, material dissolution, etc., and achieve stable electrode/electrolyte interface and high capacity retention , the effect of inhibiting the side reaction of oxygen evolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 1.1 Preparation of aqueous electrolyte
[0048] 3.64g triethyl phosphate, 2.12g LiClO 4 (anhydrous) and 0.018gH 2 O mixed (stabilizer: water: metal salt molar ratio = 2:0.1:2), heated and stirred at 80°C, and allowed to cool to room temperature to obtain an aqueous electrolyte solution.
[0049] 1.2 Performance test
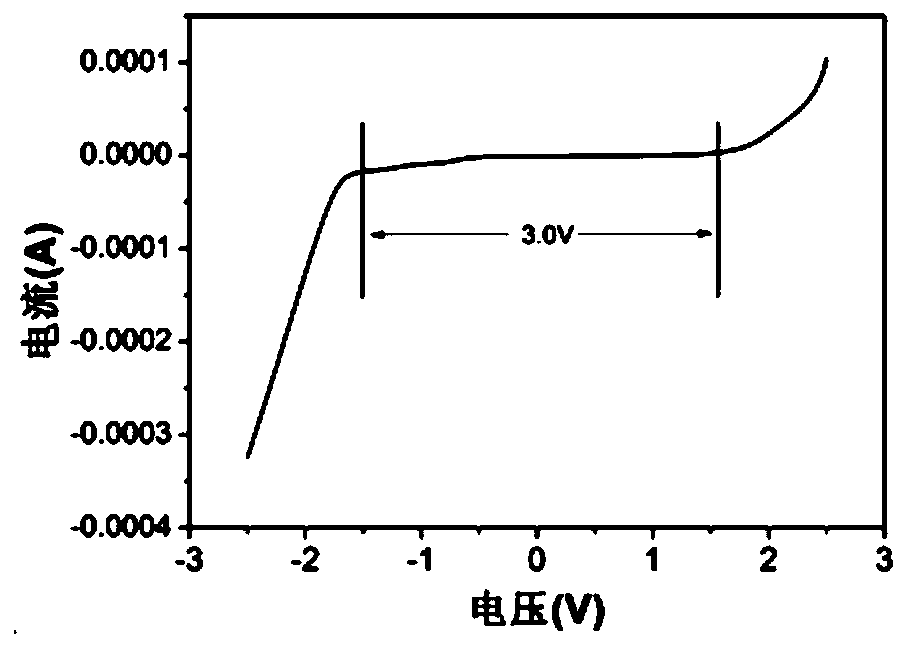
[0050] (1) Electrochemical window test
[0051] The glassy carbon electrode was used as the working electrode, the platinum wire was used as the counter electrode, and the silver-silver chloride was used as the reference electrode, and the test was carried out as a three-electrode system.
[0052] The above-mentioned three-electrode system was subjected to a cyclic voltammetry test on a Sultron (Salartron Analytical 1470E electrochemical test system in the UK), with a voltage range of -2.5V to 2.5V and a scan rate of 20mV / s.
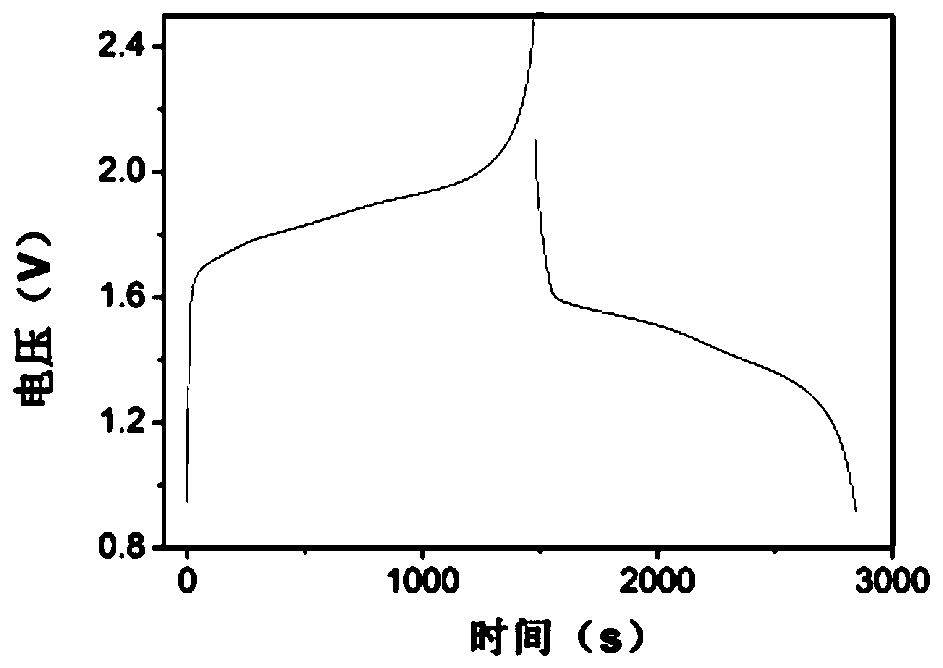
[0053] For test results see figure 1 , figure 1 From the cyclic voltammetry curve obtained in Example 1 of the present invent...
Embodiment 2~3
[0059] 1.1 Preparation of aqueous electrolyte
[0060] Prepare the aqueous electrolyte according to the preparation process of Example 1, the difference is that triethyl hemiphosphate is replaced by equimolar acetone; that is, the stabilizer is a mixture of triethyl phosphate and acetone, and the mixture of triethyl phosphate and acetone Molar ratio is 1:1, is recorded as embodiment 2.
[0061] The aqueous electrolyte solution was prepared according to the preparation process of Example 1, except that triethyl phosphate was replaced by acetone, which was recorded as Example 3.
[0062] 1.2 Performance test
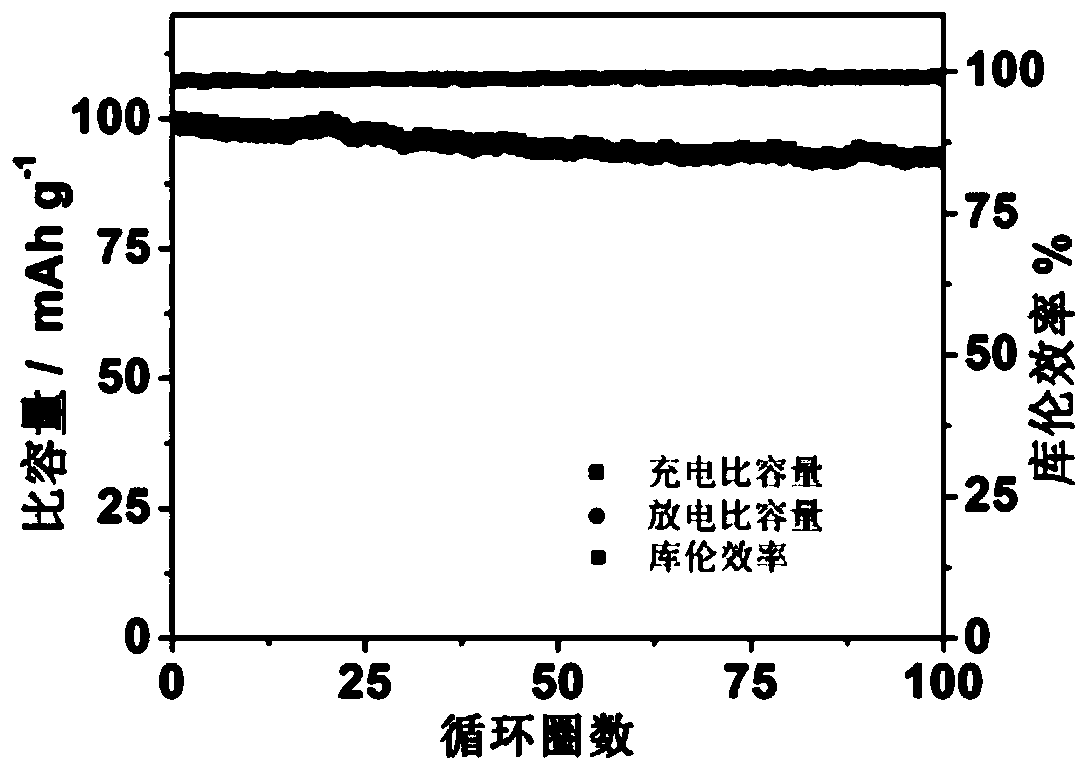
[0063] According to the test method of Example 1, the performance tests of Examples 2-3 were carried out respectively, and compared with Example 1, the results are shown in Table 1.
[0064] The performance test result of table 1 embodiment 2-6
[0065] Electrochemical window, V Charge and discharge voltage, V Capacity retention Coulombic efficiency ...
Embodiment 4~5
[0069] 1.1 Preparation of aqueous electrolyte
[0070] Prepare the aqueous electrolyte solution according to the preparation process of Example 1, the difference is that the amount of water is increased to make the molar ratio of stabilizer: water: metal salt = 2:1:2; record it as Example 4.
[0071] The aqueous electrolyte was prepared according to the preparation process of Example 1, except that the amount of stabilizer was reduced so that the molar ratio of stabilizer: water: metal salt = 1:0.1:2; recorded as Example 5.
[0072] 1.2 Performance test
[0073] According to the test method of Example 1, the performance tests of Examples 4-5 were carried out respectively, and compared with Example 1, the results are shown in Table 2.
[0074] The performance test result of table 2 embodiment 7-8
[0075] Electrochemical window, V Charge and discharge voltage, V Capacity retention Coulombic efficiency Example 1 3.0 2.5 94.6% 99.2% Example 4 2.5 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com