Catalyst for synthesizing vinyl chloride, preparation method of catalyst and vinyl chloride synthesis method
A catalyst, vinyl chloride technology, applied in the direction of organic compound/hydride/coordination complex catalysts, hydrogen halide addition preparation, chemical instruments and methods, etc., can solve the problems of cationic metal catalyst deactivation, etc., to reduce poisoning and Inactivation, good effect, no environmental pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0061] In the present invention, the preparation method of the carrier activated carbon fiber preferably comprises the following steps:
[0062] After the carbonaceous precursor and the activator are mixed, carbonization treatment is carried out to obtain activated carbon fibers.
[0063] In the present invention, the carbonaceous precursor preferably includes one or more of viscose-based fibers, polyacrylonitrile-based fibers, pitch-based fibers, phenolic-based fibers and palm fibers, when the carbonaceous precursor When multiple substances are preferred, the mass of each substance is preferably equal. In the present invention, the activator preferably includes ammonia water, phosphoric acid, diammonium hydrogen phosphate, ammonium chloride, ammonium phosphate, sodium thiosulfate, 1-(3-dimethylaminopropyl)-3-ethylcarbodi One or more of imine hydrochloride, N-hydroxysuccinimide and ethanol. When the activator is preferably multiple of the above substances, the mass of each su...
Embodiment 1
[0080] The viscose-based fibers were immersed in ammonia water with a concentration of 20wt% for 10 hours. The amount ratio of the ammonia water and viscose-based fibers was 40mL:100g. After vacuum drying, they were placed in a tube furnace. Heating at a heating rate of 5°C / min to the set temperature of 800°C, and keeping at this temperature for 3 hours for carbonization, cooling to room temperature naturally, cleaning with deionized water under ultrasonic vibration until the pH of the solution is 7, and cleaning the sample Put it into a vacuum oven for drying to obtain activated carbon fibers.
[0081] Activated carbon fiber is used as the carrier, the surface oxygen-containing groups (hydroxyl content 430μmol / g, carboxyl content 20μmol / g), ash content 1.0wt%, specific surface area 1300m 2 / g, pore volume 0.6mL / g. 4 mL of HAuCl 4 solution (where the Au content is 0.025g / mL), 6mL of CaCl 2 The solution (wherein the Ca content is 0.25g / mL) and 50mL deionized water are mixed ...
Embodiment 2
[0084] The polyacrylonitrile-based fibers are immersed in phosphoric acid with a concentration of 65wt% for 3 hours. The dosage ratio of the phosphoric acid and polyacrylonitrile-based fibers is 100mL:100g. , heated to a set temperature of 1000°C at a heating rate of 12°C / min, and kept at this temperature for 3.5 hours for carbonization, cooled naturally to room temperature, cleaned with deionized water under ultrasonic vibration until the pH of the solution = 7, and cleaned The processed samples were dried in a vacuum oven to obtain activated carbon fibers.
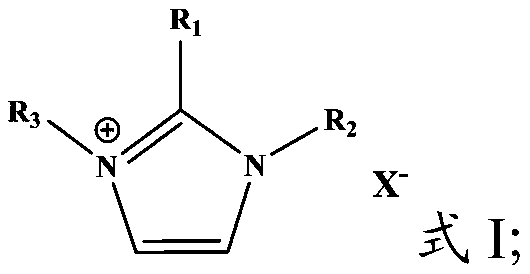
[0085] Activated carbon fiber is used as the carrier, the surface oxygen-containing groups (hydroxyl content 430μmol / g, carboxyl content 20μmol / g), ash content 1.0wt%, specific surface area 1300m 2 / g, pore volume 0.6mL / g. 4mL of RuCl 3 solution (in which the Ru content is 0.10g / mL), 1g of 1-butyl-3-methylimidazolium chloride salt and 54mL of ethanol are mixed to obtain an impregnation solution, and the impregnation so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com