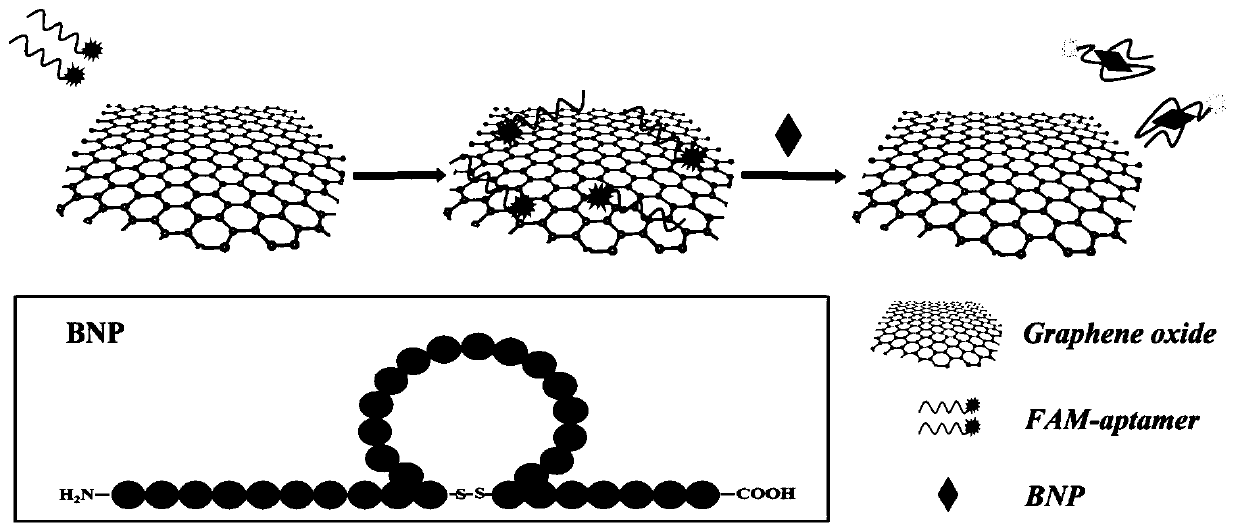
Fluorescence method for detecting brain natriuretic peptide based on graphene oxide/nucleic acid aptamer
A technology of nucleic acid aptamer and brain natriuretic peptide, which is applied in the field of medical testing, can solve the problems of detection susceptibility, susceptibility to interference, high detection cost, etc., to overcome cross-reaction interference, improve method selectivity, and high specificity detection Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] The preparation and detection method of embodiment 1 solution
[0053] Preparation of FAM-aptamer stock solution
[0054] The freeze-dried powder of FAM-aptamer was centrifuged at 6000 rpm for 5 min, and 800 μL of boiled ultrapure water was added to prepare a 2 μmol / L stock solution, which was stored in a refrigerator at 4°C. Dilute to a concentration of 100nmol / L before use.
[0055] Preparation of BNP standard stock solution
[0056] Use a pipette gun to add 2 mL of boiled ultrapure water to the lyophilized powder, and equilibrate at room temperature (20-30°C) for 15-20 minutes to completely dissolve the lyophilized powder, then gently rotate / invert the reagent until uniform, and configure The stock solution has a concentration of 1482pg / mL, stored in a -20°C refrigerator, and diluted before use.
[0057] Detection method
[0058] Blank group 1: Add 40 μL of PB buffer (pH7.4) and 15 μL of FAM-aptamer (3.75 nmol / L) in sequence to a 1.5 mL centrifuge tube, add boile...
Embodiment 2
[0062] The investigation of embodiment 2 influencing factors
[0063] The inventors studied the effect of GO concentration on the fluorescence of the FAM-aptamer probe as follows.
[0064] A series of GO solutions with different concentrations were added to the mixed solution of PB buffer (pH7.4) and FAM-aptamer (3.75nmol / L), and then the volume was adjusted to 400 μL with ultrapure water, vortexed and kept at room temperature. Stand still for 30 minutes, and measure the fluorescence parameters according to Example 1.
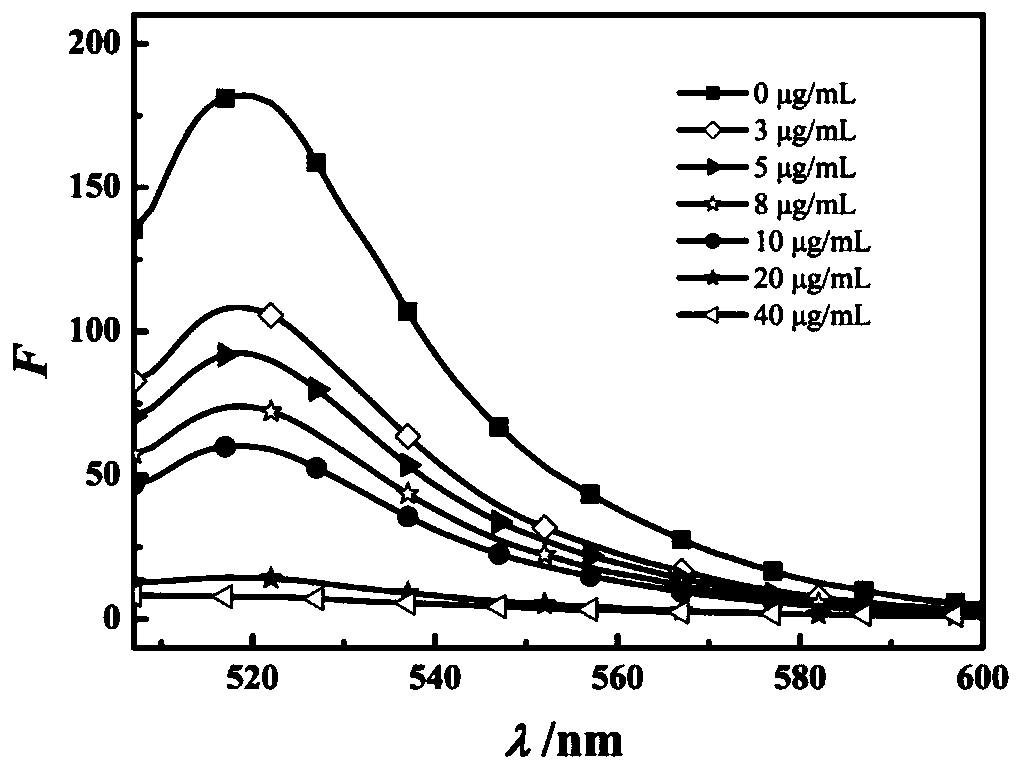
[0065] When the GO concentration is 0-40 μg / mL, the fluorescence intensity of FAM-aptamer in the system changes as follows figure 2 shown. It can be seen from the figure that when GO was added, the fluorescence of FAM-aptamer was rapidly quenched, and with the increase of GO concentration, the fluorescence intensity gradually decreased until it was completely quenched. In the present invention, 20 μg / mL GO was selected as the subsequent detection condition....
Embodiment 3
[0066] The drawing of embodiment 3 standard curve
[0067] A series of BNP standard solutions with different concentrations were added to the mixed solution consisting of 20 μL GO (400 μg / mL) and 40 μL PB buffer (pH 7.4), vortexed, allowed to stand at room temperature for 30 min, and then added to each 15 μL of FAM-aptamer (3.75 nmol / L) was added to the mixed solution, and the volume was adjusted to 400 μL with ultrapure water, vortexed to mix, and left to stand at room temperature for 30 min, and measured according to the fluorescence parameters set in Example 1. Repeat the experiment three times.
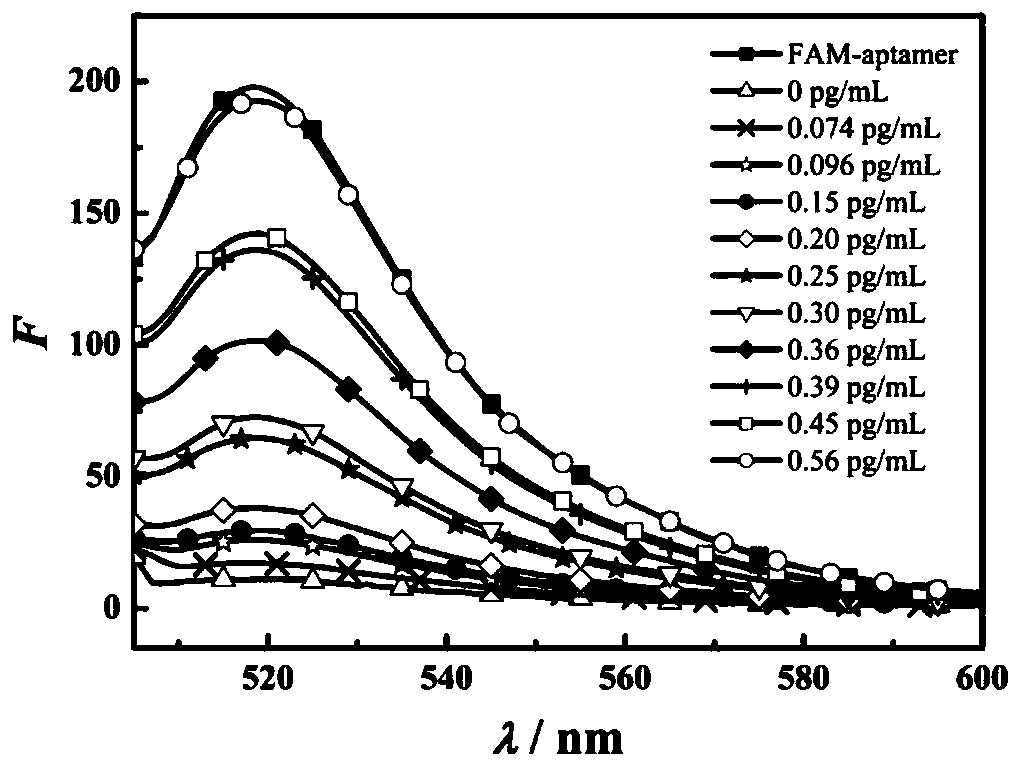
[0068] Depend on image 3 It can be seen that the fluorescence intensity of GO / FAM-aptamer composite probe gradually recovers with the increase of BNP concentration. Fluorescence recovery efficiency ((F 2 -F 1 ) / (F 0 -F 1 )) is the ordinate, the BNP concentration is the abscissa, and the fitting linearity has a good linear relationship ( Figure 4 ), the standard curve equa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com