Synthesis method of valerolactam alkaloid compound
A technology of alkaloids and valerolactam, applied in organic chemistry and other directions, can solve the problems of small amount and inability to carry out other activity research, and achieve the effects of simple operation, cheap and easily available reagents, and mild reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
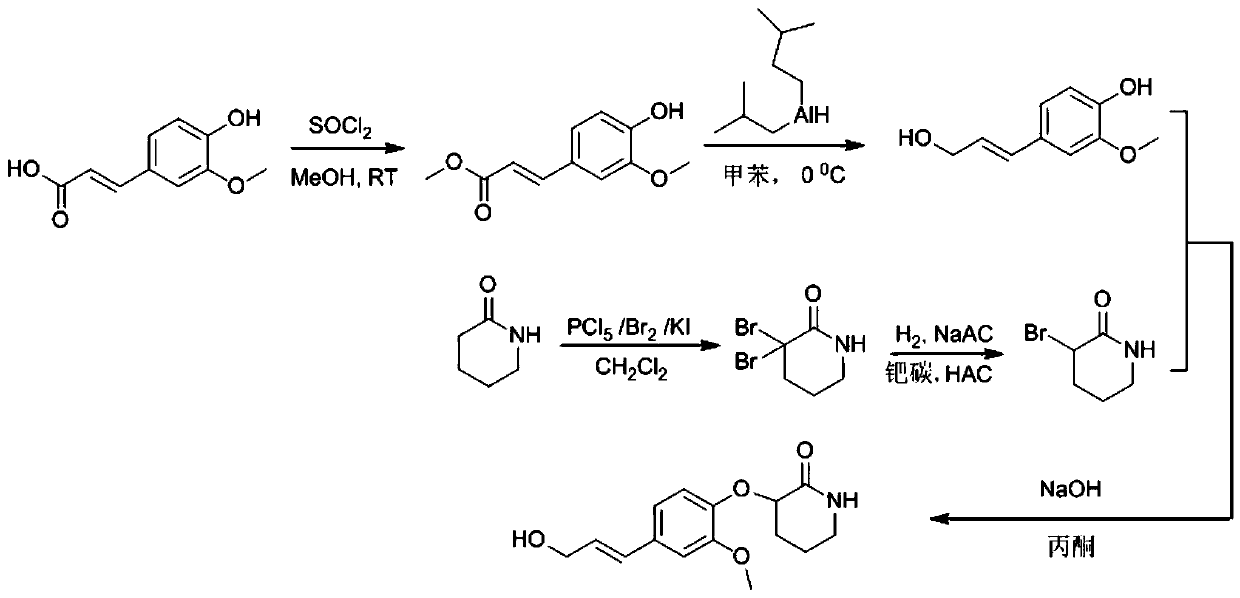
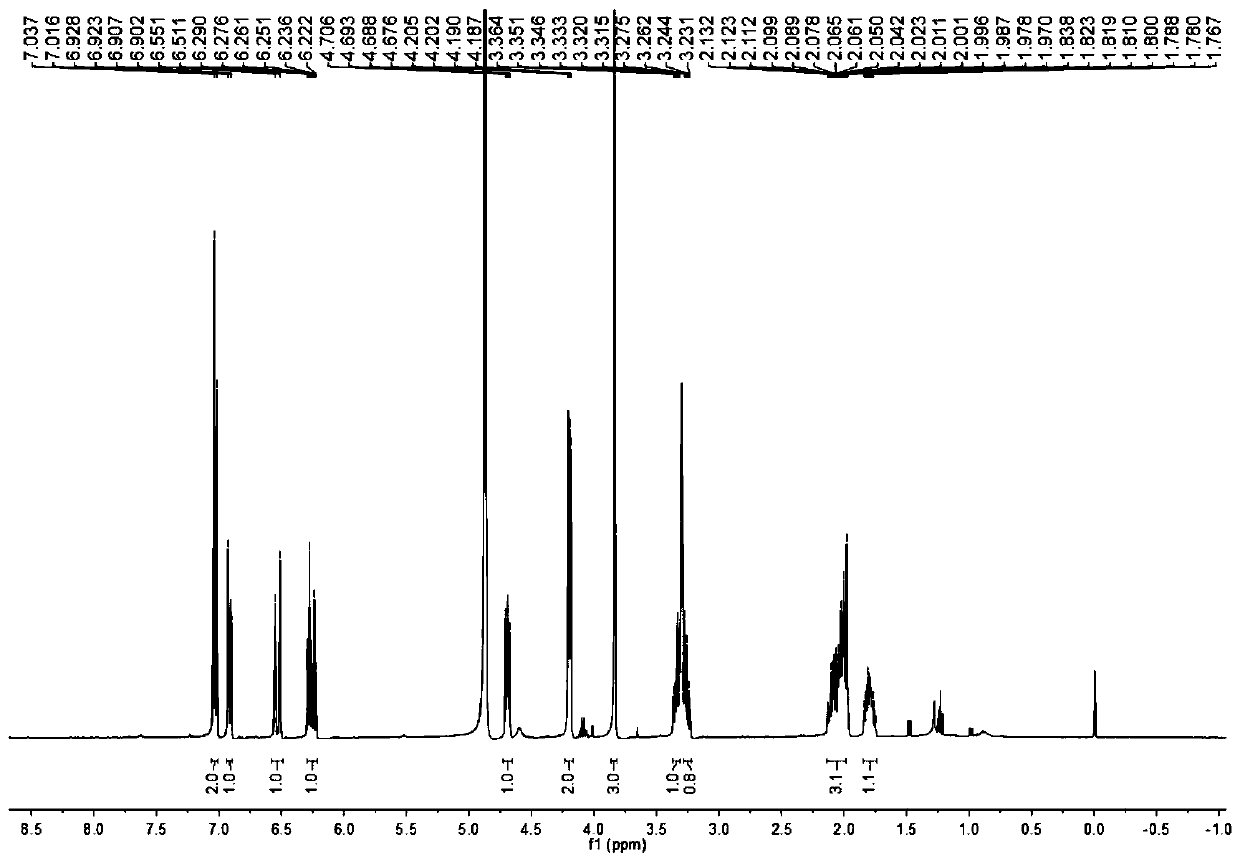
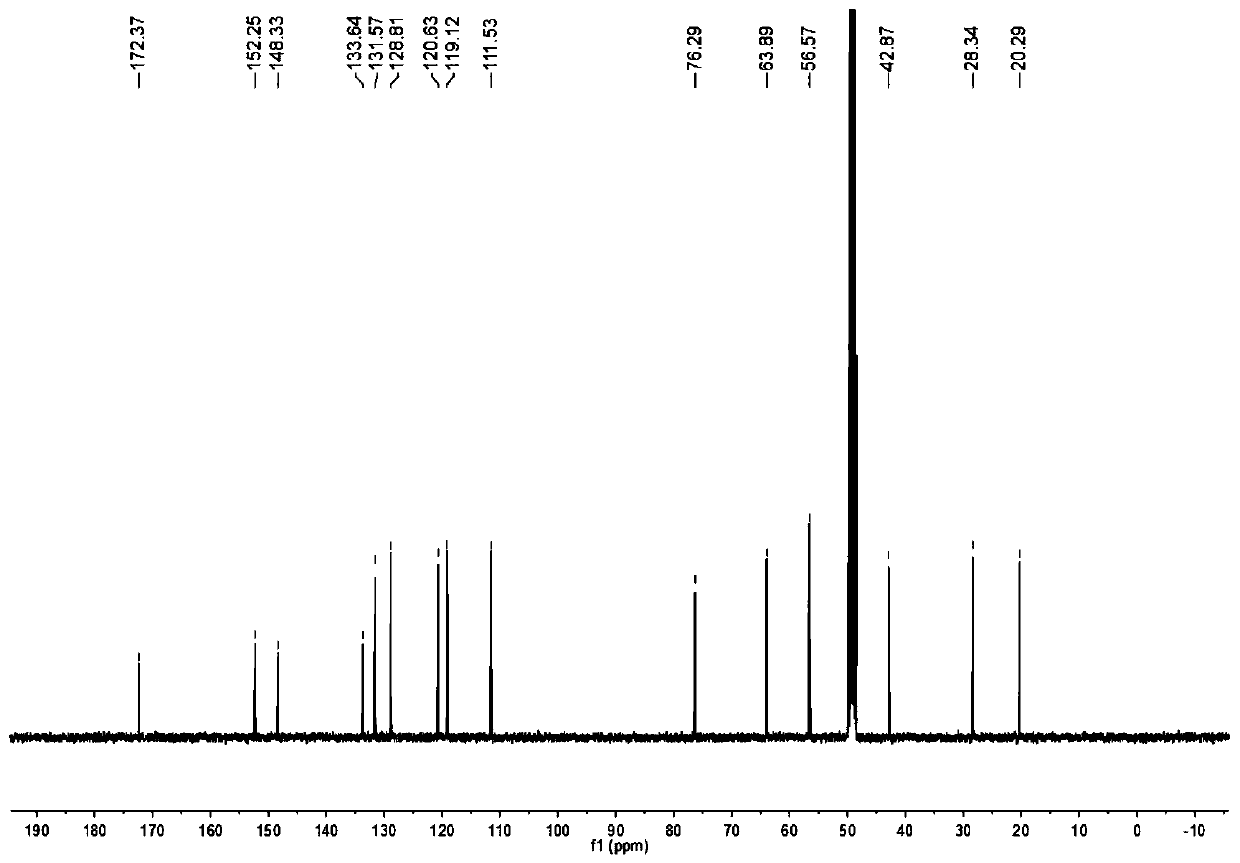
[0043] A kind of synthetic method of valerolactam alkaloid compound, comprises the following steps:
[0044] 1) ferulic acid obtains ferulic acid methyl ester by esterification
[0045] In an ice-water bath, 3 g (25.7 mmol) of thionyl chloride was added to an eggplant-shaped flask containing 50 ml of anhydrous methanol, and stirred for 30 min. Then, 5 g (25.7 mmol) of ferulic acid dissolved in 40 ml of methanol was added into the reaction system, and the reaction was stirred for 5 h.
[0046] After the reaction is complete, concentrate under reduced pressure, elute the product with 100ml ethyl acetate and then extract once with 20ml saturated brine, then dry the organic layer with anhydrous sodium sulfate, filter with suction, concentrate the filtrate, and finally use petroleum ether: ethyl acetate Esters=20:1 Passed through a silica gel column to obtain 5 g (24 mmol) of methyl ferulate, with a yield of 93.4%.
[0047] 2) Methyl ferulate is reduced by diisobutyl aluminum hyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com