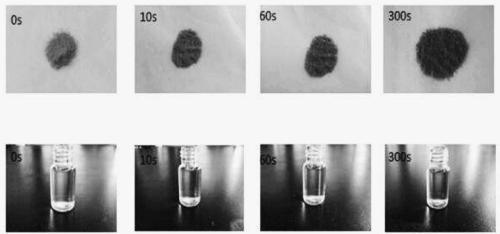
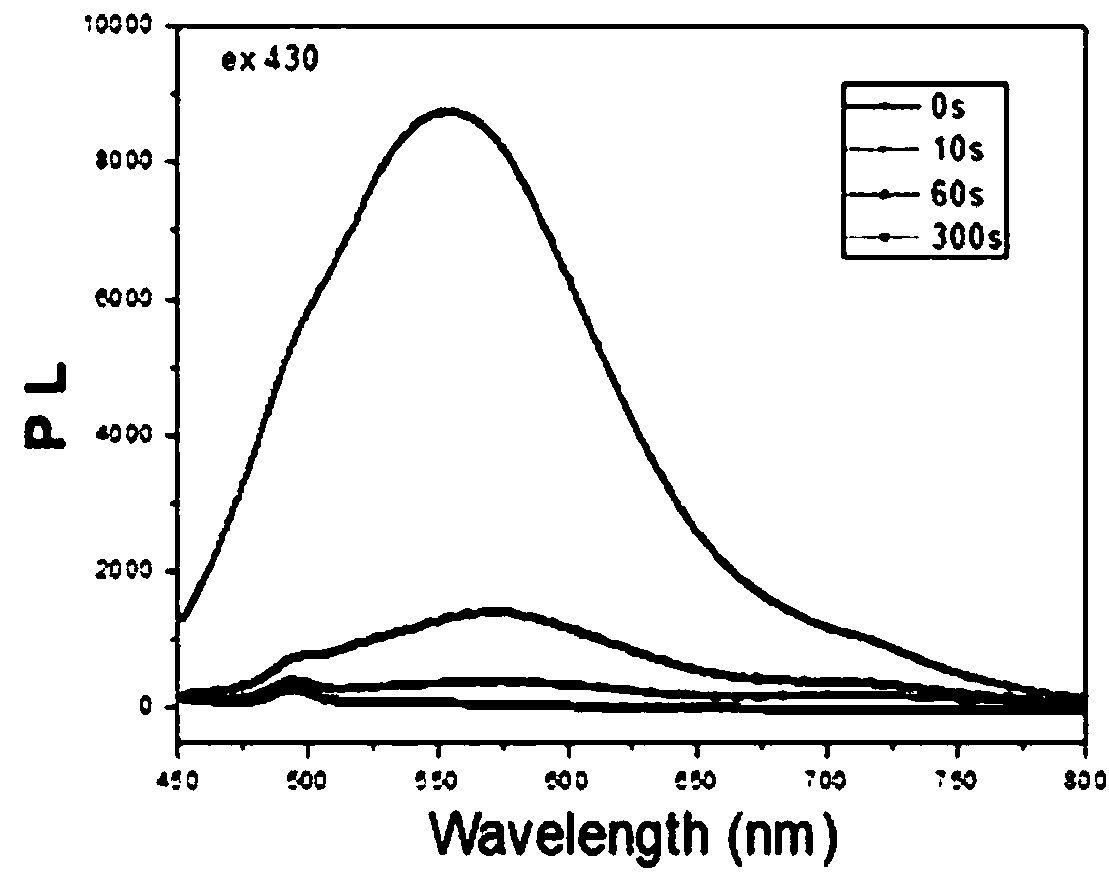
Preparation method and application of spiropyrane solid photostimulation-response compound
A compound and spiropyran technology, which is applied in the field of preparation of fluorescent stimulus-responsive compounds, can solve the problems of low isomerization efficiency and difficulty in isomerization, and achieve the effect of photo-stimulation response
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A solid-state fluorescent stimulus-responsive compound containing spiropyran and tetraphenylethylene, the specific structural formula of which is as follows:
[0024]
[0025] The preparation method is as follows:
[0026] (1) Synthesis of 2,3,3-trimethyl-N-hydroxyethylindole (compound A)
[0027]
[0028] Take by weighing 2,3,3-trimethyl-3H-indole (2.61g, 16mmol) and 2-bromoethanol (2.46g, 20mmol) and join in the three-necked flask of 100ml, then add solvent acetonitrile (20ml), pack With a good reflux device, under the protection of nitrogen, heat and reflux for 24 hours (oil bath 80 degrees), spin dry the solvent, wash with n-hexane three times, and then dry under reduced pressure to obtain 3.45 g of a purple solid with a yield of 75%. 1 H NMR (400MHz, CDCl 3 ), chemical shift (ppm): 7.93(t,1H,Ar-H),7.84(d,1H,Ar-H),7.60(t,1H,Ar-H),7.58,(d,1H,Ar-H H),4.57(t,2H,-NCH 2 CH 2 -O-),3.85(m,2H,-NCH 2 CH 2 -O-), 3.35(s,1H,-OH), 1.37(s,3H,C(N)(O)CH 3 ),1.31(s,3H,...
Embodiment 2
[0042] A solid-state fluorescent stimulus-responsive compound containing spiropyran and tetraphenylethylene, the specific structural formula of which is as follows:
[0043]
[0044] The preparation method is as follows:
[0045] Compound A, Compound B, Compound C, and synthetic steps refer to Example 1;
[0046] (1) Synthesis of Compound D
[0047] Weigh compound C (3g, 8.49mmol) and 1,2-dibromoethane (2.39g, 12.74mmol) into a 100ml three-necked flask, then add potassium carbonate (3.51g, 25.47mmol) and acetone (80ml) , install the reflux device, and heat to reflux for 24 hours under the protection of nitrogen. The solvent was spin-dried, and the crude product was purified by column chromatography (silica gel) using petroleum ether and ethyl acetate (6:1) as the eluent to obtain 2.69 g of light yellow solid powder with a yield of 70%.
[0048] (2) Synthesis of target product 2
[0049] Weigh compound D (1.5g, 3.26mmol) and tetraphenylethylene (0.39g, 1.08mmol) and add ...
Embodiment 3
[0051] A solid-state fluorescent stimulus-responsive compound containing spiropyran and tetraphenylethylene, the specific structural formula of which is as follows:
[0052]
[0053] The preparation method is as follows:
[0054] For the synthesis steps of compound A, compound B, compound C and compound D, see Example 1;
[0055] (1) Synthesis of Compound D
[0056] Weigh compound C (2.51g, 7.11mmol) and 1,2-dibromohexane (2.61g, 10.66mmol) into a 100ml three-necked flask, then add potassium bicarbonate (2.33g, 23.31mmol) and acetone (80ml), equipped with a reflux device, under nitrogen protection, heated to reflux for 24 hours. The solvent was spin-dried, and the crude product was purified by column chromatography (silica gel) using petroleum ether and ethyl acetate (6:1) as the eluent to obtain 2.48 g of light yellow solid powder with a yield of 68%.
[0057] (2) Synthesis of target product 3
[0058] Weigh compound D (2.2g, 4.27mmol) and tetraphenylethylene (0.517g, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com