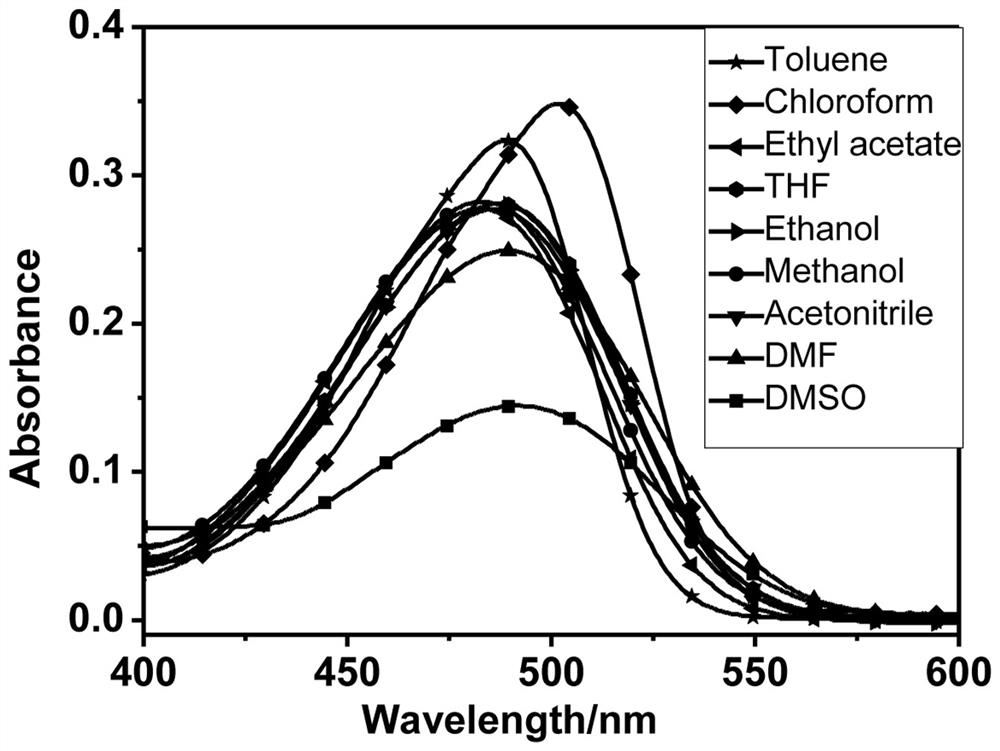
A kind of reactive hydrazine fluorescent probe and its preparation method and application
A fluorescent probe and reactive technology, which is applied in the detection trap fluorescent probe and its preparation field, can solve the problems of complex probe synthesis process, weak anti-interference, narrow linear range, etc., and achieve high yield and post-processing Convenience, compositing simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Preparation of 2-(2-chloro-7-diethylaminoquinolin-3-yl)methylenemalononitrile (QAM)
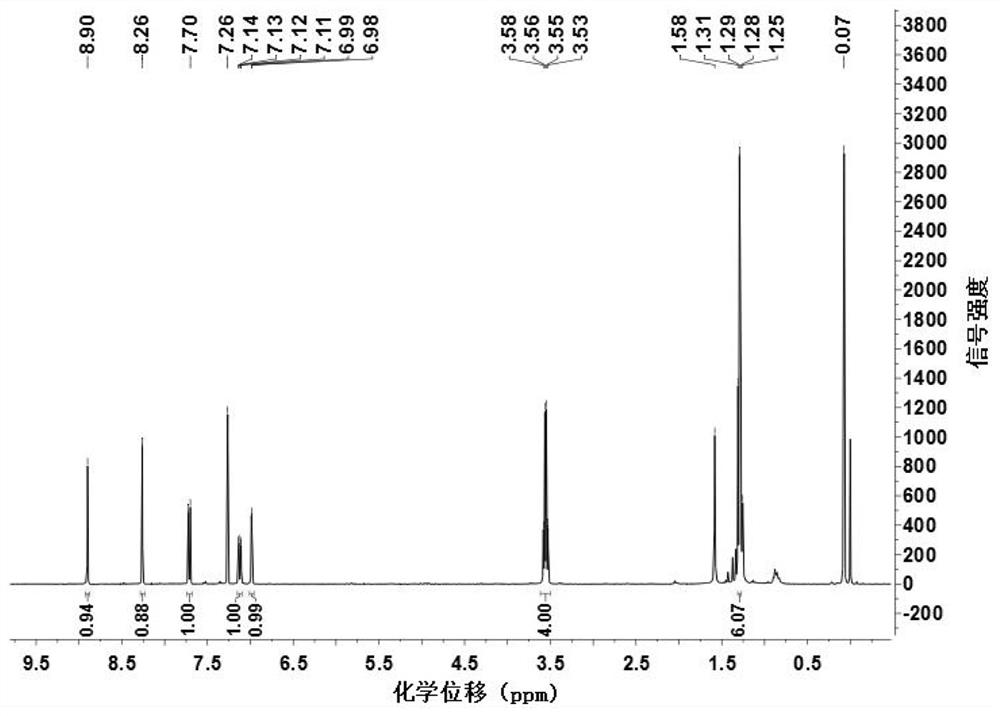
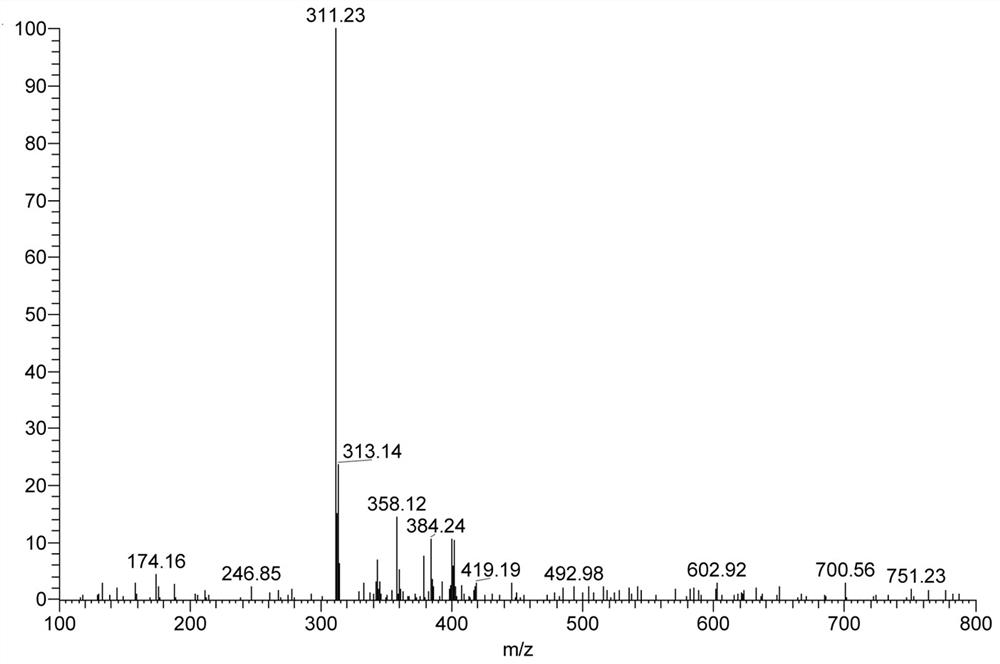
[0048] Add 0.23g (0.88mmol) 2-chloro-7-diethylaminoquinoline-3-carboxaldehyde, 0.10g (1.51mmol) propanedinitrile, add 4.00mL absolute ethanol, 0.2mL pyridine, 0.1 mL of glacial acetic acid was stirred under reflux until the reaction of 2-chloro-7-diethylaminoquinoline-3-carbaldehyde was complete (TLC tracking), cooled to room temperature, filtered with suction, washed with a small amount of ethanol, and dried to obtain 0.23 g of a red solid. Namely 2-(2-chloro-7-diethylaminoquinolin-3-yl)methylene malononitrile (QAM), yield 82.50%, 1 H NMR (400MHz, CDCl 3)δ8.90(s, 1H), 8.26(s, 1H), 7.71(d, J=9.3Hz, 1H), 7.12(dd, J=9.3, 2.6Hz, 1H), 6.98(d, J=2.4 Hz, 1H), 3.55 (q, J=7.1Hz, 4H), 1.29 (t, J=7.1Hz, 6H). IR(KBr), ν / cm-1:2226,1623. ESI-Ms: 311.23 (M+1).
Embodiment 2
[0050] Preparation of 2-(2-chloro-7-diethylaminoquinolin-3-yl)methylenemalononitrile (QAM)
[0051] Add 0.18g (0.69mmol) 2-chloro-7-diethylaminoquinoline-3-carbaldehyde, 0.06g (0.91mmol) acetonitrile, 3.50mL absolute ethanol, 0.2mL glacial acetic acid into a 50mL two-necked bottle, Reflux and stir for 14 hours, cool to room temperature, filter with suction, wash with a small amount of ethanol, and dry to obtain 0.19 g of a red solid. That is, 2-(2-chloro-7-diethylaminoquinolin-3-yl)methylenemalononitrile (QAM), with a yield of 88.83%. 1 H-NMR and ESI-MS characterization data are the same as in Example 1.
Embodiment 3
[0053] Preparation of 2-(2-chloro-7-diethylaminoquinolin-3-yl)methylenemalononitrile (QAM)
[0054] Add 0.18g (0.69mmol) 2-chloro-7-diethylaminoquinoline-3-carbaldehyde, 0.06g (0.91mmol) propanedinitrile, 3.50mL absolute ethanol, 0.1mL pyridine, and reflux in a 50mL two-necked bottle. Stir for 14 h, cool to room temperature, filter with suction, wash with a small amount of ethanol, and dry to obtain 0.18 g of a red solid. That is, 2-(2-chloro-7-diethylaminoquinolin-3-yl)methylenemalononitrile (QAM), with a yield of 84.15%. 1 H-NMR and ESI-MS characterization data are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com