Graphite phase carbon nitride and preparation method thereof, and hydrogen production photocatalyst and applications thereof
A graphitic carbon nitride and photocatalyst technology, applied in physical/chemical process catalysts, chemical instruments and methods, nitrogen compounds, etc., can solve problems such as limiting the efficiency of photocatalytic hydrogen production, and achieve good visible light response and good visible light catalysis Hydrogen production activity and the effect of enriching photocatalytic hydrogen production active sites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
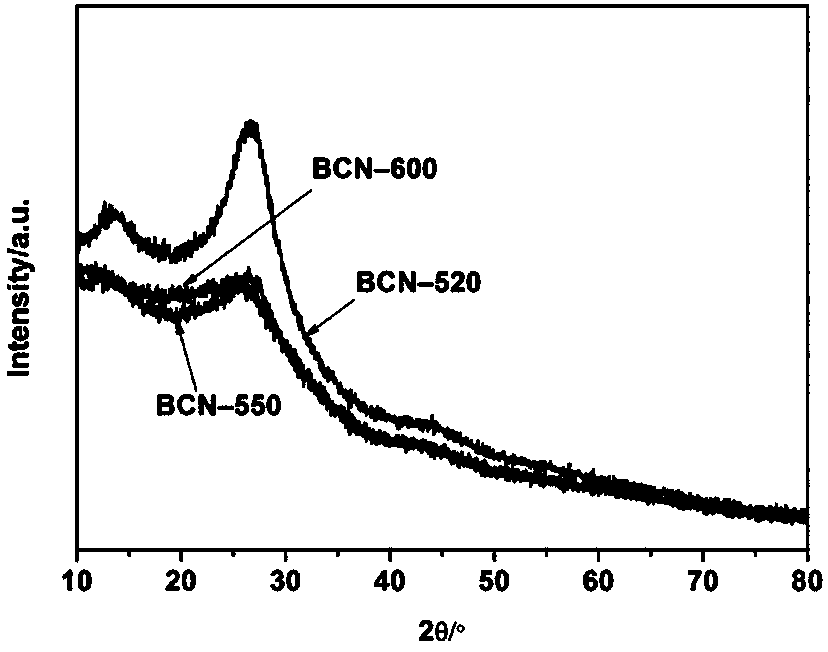
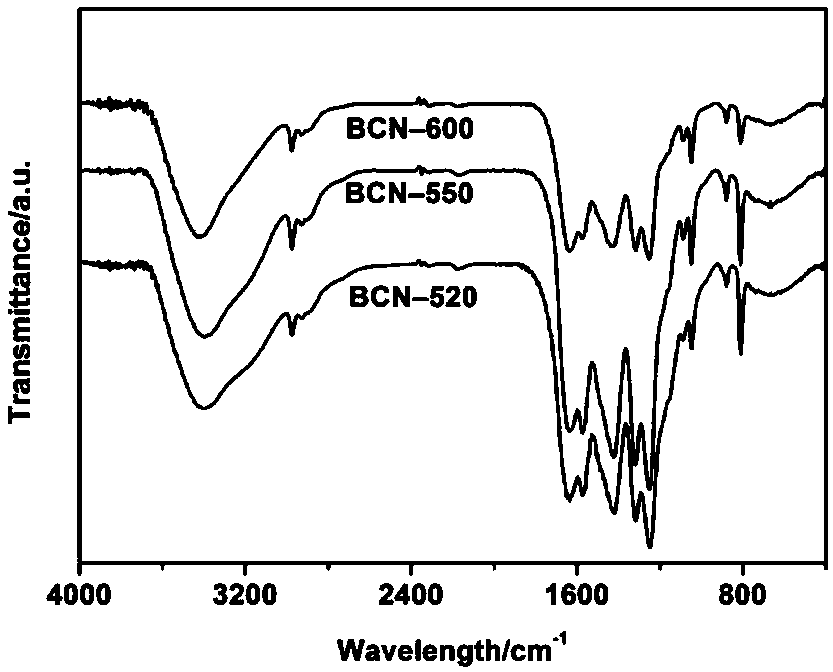
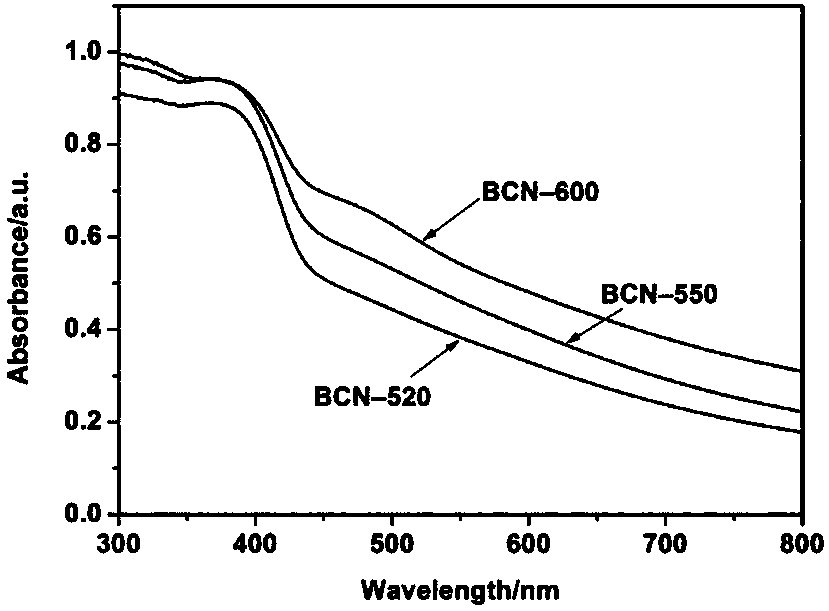
[0032] Step 1: Weigh 1.0g of biuret, place it in a small crucible, put the small crucible in a tube furnace, and pass argon into the tube furnace for half an hour to drive away the air in the furnace. Then set the program to raise the temperature to 520°C at 5°C / min and hold it for 4h, and finally cool naturally to obtain a new type of graphite phase carbon nitride, which is named BCN-520.
[0033] Step 2: Add the graphite-phase carbon nitride powder prepared in step 1 into the reaction system for photocatalytic water splitting to produce hydrogen, and load platinum (loaded with 3wt% platinum) by photoreduction method to conduct photocatalytic water splitting hydrogen production test. Specific steps are as follows:
[0034] 1) Add 10.0 mg of graphitic carbon nitride into a reactor with a volume of 100 mL, and add a total volume of 80 mL of an aqueous solution with a triethanolamine content of 10 vol% as a sacrificial agent; and add 0.425 mL of platinum with a content of 0.0007...
Embodiment example 2
[0039] Step 1: Weigh 1.0g of biuret, place it in a small crucible, put the small crucible in a tube furnace, and pass argon into the tube furnace for half an hour to drive away the air in the furnace. Then set the program to raise the temperature to 550°C at 5°C / min and hold it for 4h, and finally cool naturally to obtain a new type of graphite phase carbon nitride, which is named BCN-550.
[0040] Step 2: Add the graphite-phase carbon nitride powder prepared in step 1 into the reaction system for photocatalytic water splitting to produce hydrogen, and load platinum (loaded with 3wt% platinum) by photoreduction method to conduct photocatalytic water splitting hydrogen production test. Specific steps are as follows:
[0041] 1) Add 10.0 mg of graphitic carbon nitride into a reactor with a volume of 100 mL, and add a total volume of 80 mL of an aqueous solution with a triethanolamine content of 10 vol% as a sacrificial agent; and add 0.425 mL of platinum with a content of 0.0007...
Embodiment example 3
[0046]Step 1: Weigh 1.0g of biuret, place it in a small crucible, put the small crucible in a tube furnace, and pass argon into the tube furnace for half an hour to drive away the air in the furnace. Then set the program to raise the temperature to 600°C at 5°C / min and hold it for 4h, and finally cool naturally to obtain the prepared new graphite phase carbon nitride, which is named BCN-600.
[0047] Step 2: Add the graphite-phase carbon nitride powder prepared in step 1 into the reaction system for photocatalytic water splitting to produce hydrogen, and load platinum (loaded with 3wt% platinum) by photoreduction method to conduct photocatalytic water splitting hydrogen production test. Specific steps are as follows:
[0048] 1) Add 10.0 mg of graphitic carbon nitride into a reactor with a volume of 100 mL, and add a total volume of 80 mL of an aqueous solution with a triethanolamine content of 10 vol% as a sacrificial agent; and add 0.425 mL of platinum with a content of 0.00...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com