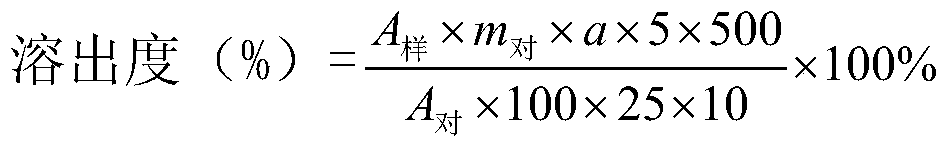Domperidone orally disintegrating tablet and preparing method thereof
A technology for oral disintegrating tablets and domperidone, which is applied in the directions of pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc., can solve the problems of gritty feeling, large difference in tablet weight, sticking and flushing, etc., To achieve the effect of improving fluidity, quality assurance, and improving sticking and punching
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
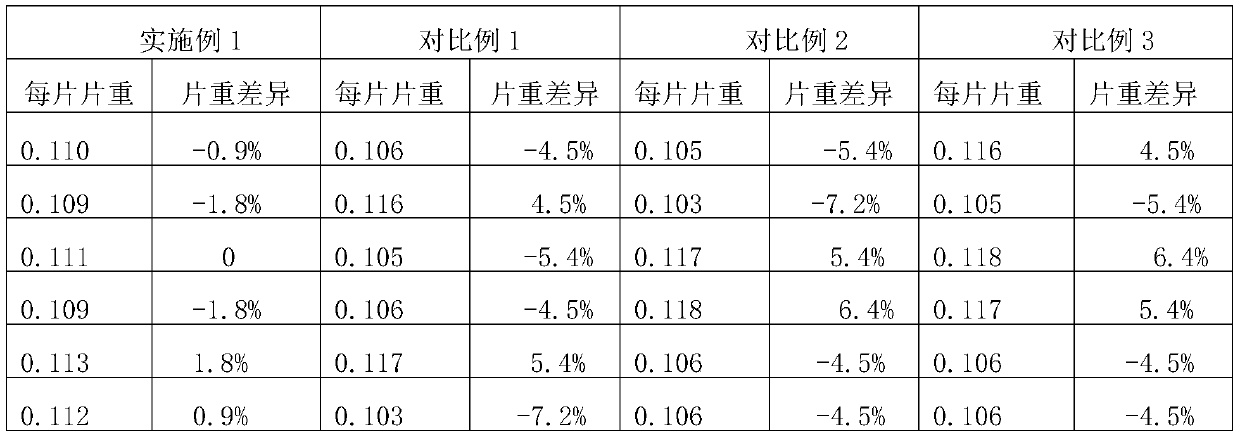
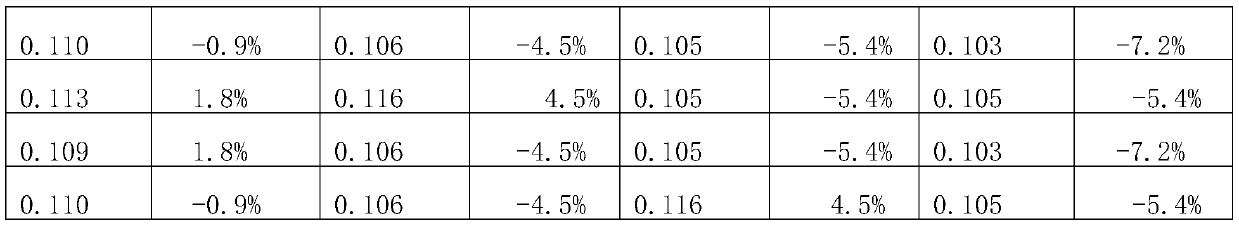
[0023] An embodiment of the domperidone orally disintegrating tablet of the present invention comprises the following components by weight: 10 parts by weight of domperidone, 80 parts by weight of filler, 10 parts by weight of disintegrant, 5 parts by weight of sweetener, flow aid 2.1 parts by weight of agent, 0.54 parts by weight of lubricant. The filler is microcrystalline cellulose, the disintegrant is croscarmellose sodium, the sweetener is aspartame, and the glidant is silicon dioxide. Described lubricant is the mixture of magnesium stearate, glyceryl behenate, sodium stearyl fumarate, and the weight ratio of described magnesium stearate, glyceryl behenate, sodium stearyl fumarate is 3: 1:2.
[0024] The preparation method of above-mentioned domperidone orally disintegrating tablet, comprises the following steps:
[0025] (1) After pulverizing the domperidone, filler, disintegrant, sweetener and glidant respectively, pass through a 100-mesh sieve respectively;
[0026]...
Embodiment 2
[0030] An embodiment of the domperidone orally disintegrating tablet of the present invention comprises the following components by weight: 10 parts by weight of domperidone, 80 parts by weight of filler, 10 parts by weight of disintegrant, 5 parts by weight of sweetener, flow aid 2.1 parts by weight of agent, 0.54 parts by weight of lubricant. The filler is microcrystalline cellulose, the disintegrant is croscarmellose sodium, the sweetener is aspartame, and the glidant is silicon dioxide. Described lubricant is the mixture of magnesium stearate, glyceryl behenate, sodium stearyl fumarate, and the weight ratio of described magnesium stearate, glyceryl behenate, sodium stearyl fumarate is 1: 1:4.
[0031] The preparation method of above-mentioned domperidone orally disintegrating tablet, comprises the following steps:
[0032] (1) After pulverizing the domperidone, filler, disintegrant, sweetener and glidant respectively, pass through a 100-mesh sieve respectively;
[0033]...
Embodiment 3
[0037] An embodiment of the domperidone orally disintegrating tablet of the present invention comprises the following components by weight: 10 parts by weight of domperidone, 80 parts by weight of filler, 10 parts by weight of disintegrant, 5 parts by weight of sweetener, flow aid 2.1 parts by weight of agent, 0.54 parts by weight of lubricant. The filler is microcrystalline cellulose, the disintegrant is croscarmellose sodium, the sweetener is aspartame, and the glidant is silicon dioxide. Described lubricant is the mixture of magnesium stearate, glyceryl behenate, sodium stearyl fumarate, and the weight ratio of described magnesium stearate, glyceryl behenate, sodium stearyl fumarate is 1: 5:2.
[0038] The preparation method of above-mentioned domperidone orally disintegrating tablet, comprises the following steps:
[0039] (1) After pulverizing the domperidone, filler, disintegrant, sweetener and glidant respectively, pass through a 100-mesh sieve respectively;
[0040]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com