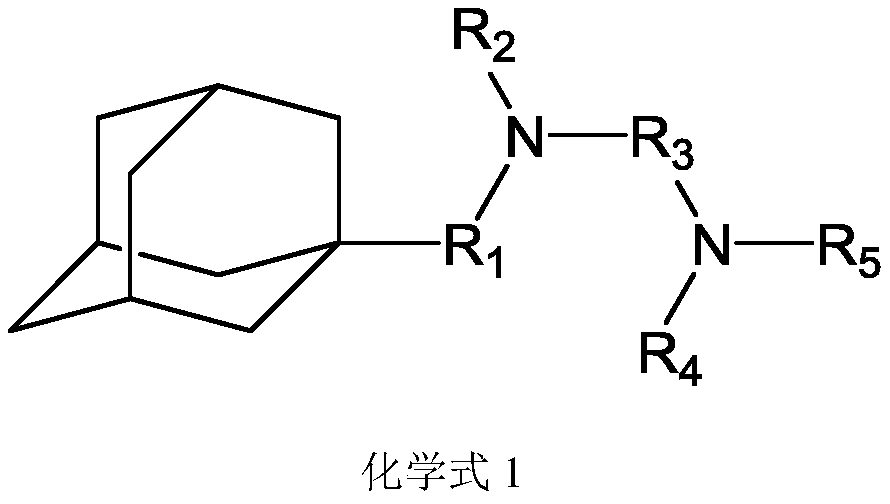
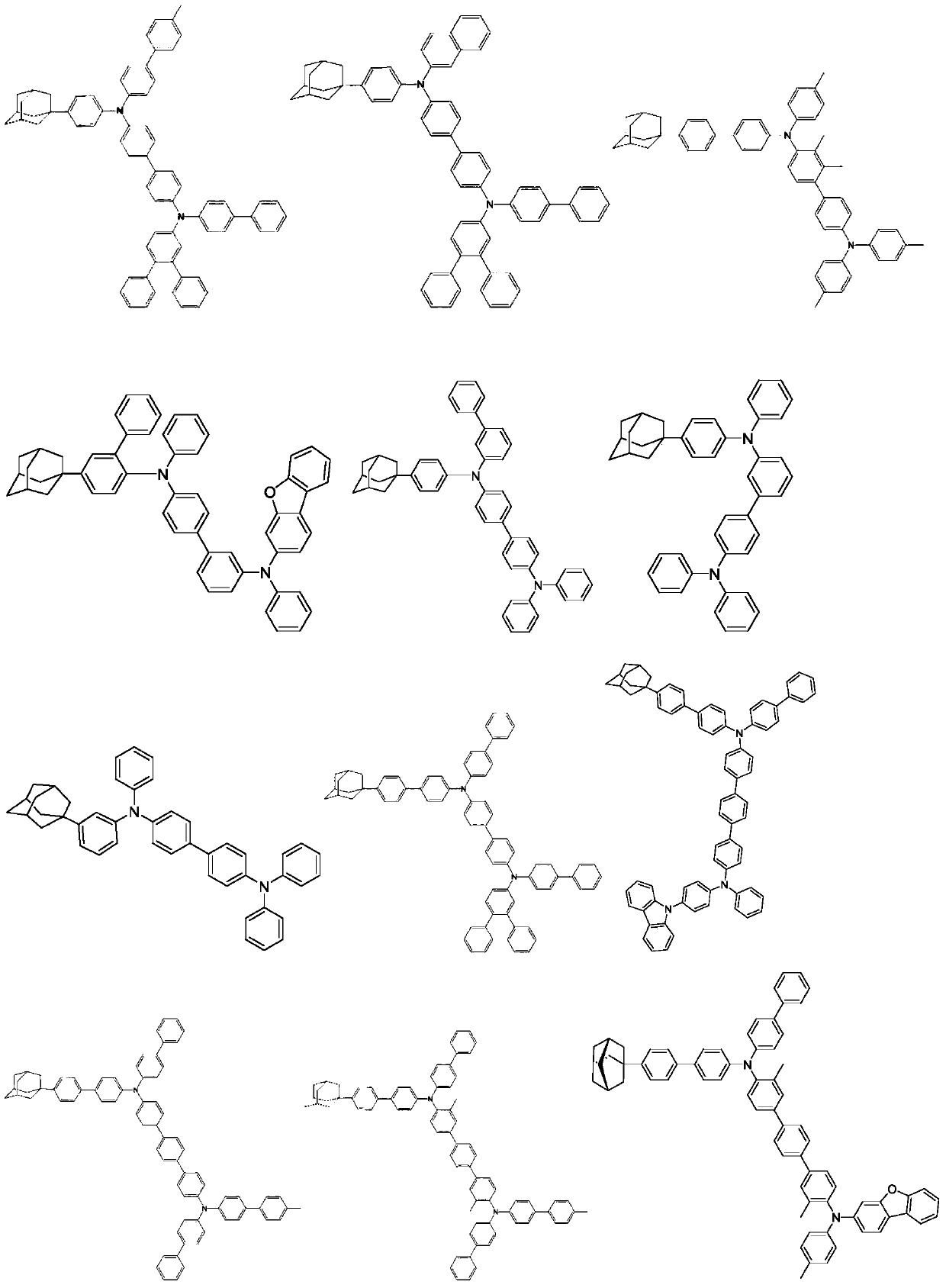
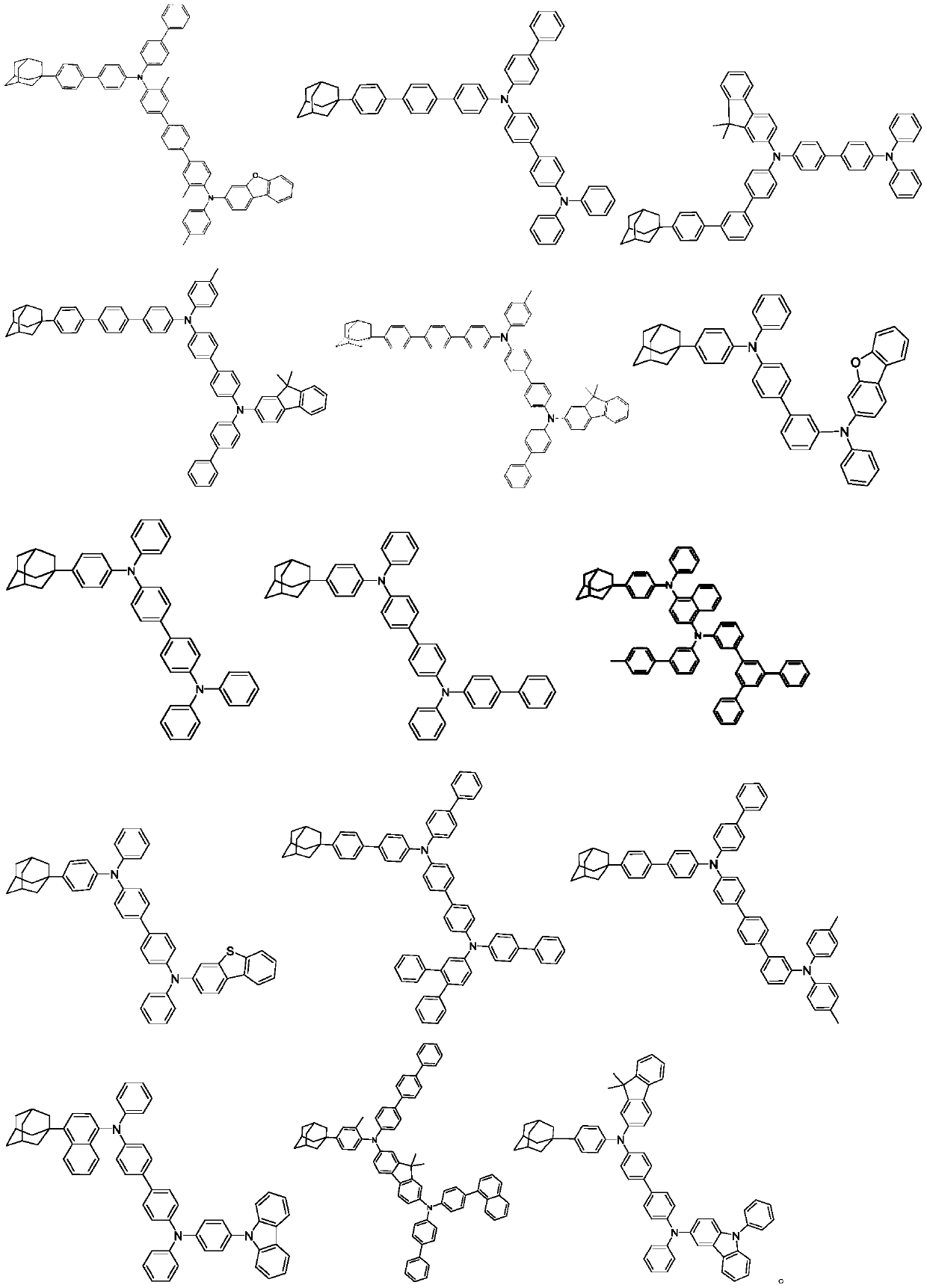
An organic electroluminescent material and an organic electroluminescent device comprising the same
An electroluminescent material and luminescent technology, applied in luminescent materials, electric solid devices, organic chemistry, etc., can solve the problems of easy crystallization and performance degradation of light-emitting devices, and achieve optimal hole transport rate adjustment and high luminous efficiency and service life, the effect of low driving voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0041] Synthesis of compound 1
[0042]
[0043] Add 1-adamantanol (50.0g, 328.4mmol), bromobenzene (51.6g, 328.4mmol), dichloromethane (500mL) into a round bottom flask, cool down to -5-0°C under nitrogen protection, and dropwise add Trifluoromethanesulfonic acid (73.9g, 492.6mmol), keep stirring for 3h; add deionized water (300mL) to the reaction solution and wash to pH=7, add dichloromethane (100mL) for extraction, combine the organic phases, use Dry over magnesium sulfate, filter, and remove the solvent under reduced pressure; the resulting crude product is purified by silica gel column chromatography using n-heptane as the mobile phase to obtain white solid intermediate I-A-1 (53.1 g, 55.4%).
[0044]
[0045] Intermediate I-A-1 (7.0g, 24.04mmol), aniline (2.69g, 28.84mmol), tris(dibenzylideneacetone) dipalladium (0.22g, 0.24mmol), 2-dicyclohexylphosphorus-2' , 4',6'-triisopropylbiphenyl (0.23g, 0.48mmol) and sodium tert-butoxide (3.46g, 36.05mmol) were added to to...
Synthetic example 2
[0051] Synthesis of Compound 2
[0052]
[0053] N-phenyl-4-benzidine (5.00g, 20.38mmol), 4-bromo-4'-iodobiphenyl (9.15g, 25.48mmol), tris(dibenzylideneacetone)dipalladium (0.19g, 0.20mmol), tri-tert-butylphosphine (0.08g, 0.41mmol) and sodium tert-butoxide (2.94g, 30.57mmol) were added to toluene (80mL), heated to 108°C under nitrogen protection, stirred for 16h; then cooled to room temperature , the reaction solution was washed with water and dried by adding magnesium sulfate. After filtration, the filtrate was desolventized under reduced pressure; using n-heptane as the mobile phase for silica gel column chromatography to obtain intermediate II-B (5.55g, 57.16%).
[0054]
[0055] Intermediate I-A (4.70g, 15.49mmol), intermediate II-B (5.55g, 15.49mmol), tris(dibenzylideneacetone)dipalladium (0.14g, 0.15mmol), 2 -Dicyclohexylphosphonium-2', 6'-dimethoxybiphenyl (0.13g, 0.31mmol) and sodium tert-butoxide (2.23g, 23.23mmol) were added to toluene (50mL), and heated to 1...
Synthetic example 3
[0057] Synthesis of Compound 3
[0058]
[0059] 3-Bromodibenzofuran (10.0g, 40.47mmol), aniline (4.15g, 44.52mmol), tris(dibenzylideneacetone) dipalladium (0.37g, 0.40mmol), 2-dicyclohexylphosphine- 2,4,6-Triisopropylbiphenyl (0.39g, 0.81mmol) and sodium tert-butoxide (5.84g, 60.71mmol) were added to toluene (100mL), heated to 108°C under nitrogen protection, and stirred for 1h; then Cool to room temperature, wash the reaction solution with water, add magnesium sulfate to dry, filter and pass the filtrate through a short silica gel column, and remove the solvent after passing through the column under reduced pressure; use dichloromethane / n-heptane system to recrystallize and purify the crude product to obtain a gray solid Intermediate II-C-1 (8.2 g, 78.17%).
[0060]
[0061] 3-Bromo-4'chloro-1,1'-biphenyl (8.00g, 29.90mmol), intermediate II-C-1 (5.17g, 19.93mmol), tris(dibenzylideneacetone)dipalladium ( 0.18g, 0.20mmol), tri-tert-butylphosphine (0.10g, 0.40mmol) and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| glass transition temperature | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com