Apparatus and method for preparing high-purity lithium fluoride
A high-purity lithium fluoride and grinding device technology, applied in chemical instruments and methods, lithium halides, feeding devices, etc., can solve problems such as not considering the corrosion requirements of hydrofluoric acid, and achieve the effect of avoiding corrosion and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
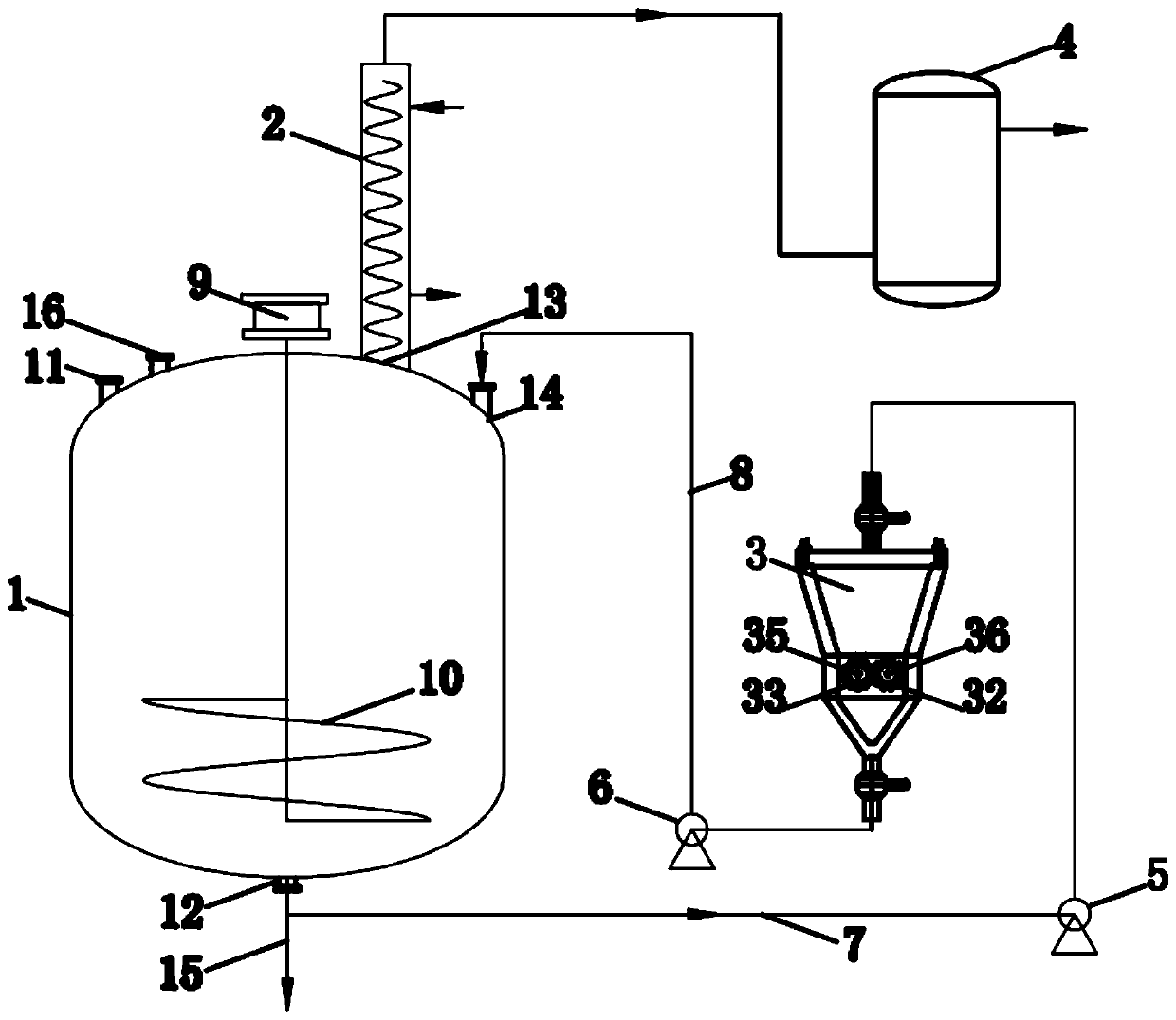
[0023] figure 1 and figure 2 A device for preparing high-purity lithium fluoride according to an embodiment of the present invention is schematically shown.
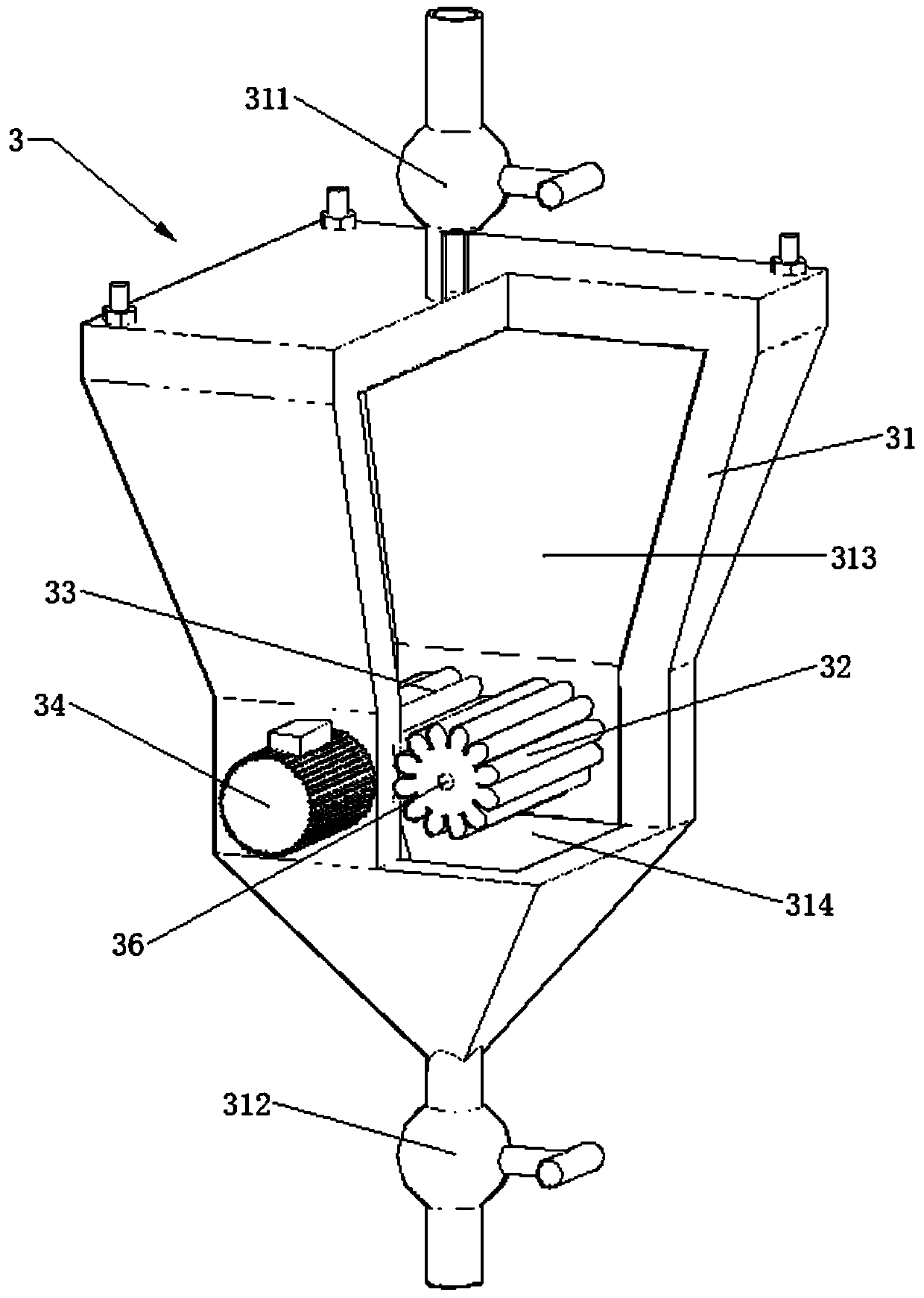
[0024] refer to figure 1 and figure 2 , a device for preparing high-purity lithium fluoride Reactor 1, condensation reflux device 2, grinding device 3, tail gas absorption device 4, first corrosion-resistant pump 5, second corrosion-resistant pump 6, first return pipe 7, the second Two return pipes 8, motors 9 and stirring paddles 10.
[0025] The reaction kettle 1 is made of stainless steel, and the reaction kettle 1 is lined with polytetrafluoroethylene, which can effectively prevent hydrofluoric acid corrosion. The reaction kettle 1 is provided with a solid feed port 11, a liquid feed port 16, a discharge port 12, a condensation return port 13 and a mixed slurry return port 14, and the feed port includes a solid feed port 11 and a liquid feed port 16, The solid feed port 11 , the liquid feed port 16 , the conde...
Embodiment 2
[0033] The present invention also provides a method for preparing high-purity lithium fluoride, comprising the steps of:
[0034] 1) Lithium carbonate solid particles and hydrofluoric acid liquid are mixed in reactor 1, violent reaction takes place under the stirring of paddle 10 and has generated lithium fluoride-hydrofluoric acid-lithium carbonate mixed slurry and carbon dioxide gas;
[0035] The chemical reaction equation of this reaction is: Li 2 CO 3 +2HF=2LiF↓+CO 2 ↑+H 2 O.
[0036]Due to the violent reaction, hydrogen fluoride gas will be volatilized in the hydrofluoric acid liquid raw material, and the hydrogen fluoride gas will mix with the carbon dioxide gas generated by the reaction and overflow together.
[0037] The lithium fluoride produced by the reaction will be wrapped on the surface of lithium carbonate crystal, hindering the further reaction of lithium carbonate and hydrofluoric acid. Therefore, in the prior art, this reaction process usually cannot mak...
Embodiment 3
[0046] Weigh 100g of lithium carbonate dried to constant weight for later use, connect the pipeline of the device, open the condensation reflux device 2 (condensation medium is absolute ethanol, temperature -30°C), weigh 56.87g of anhydrous hydrogen fluoride (5% excess) and place in In the reaction kettle 1, the sodium carbonate weighed was slowly added into the reaction kettle, and the first corrosion-resistant pump 5, the second corrosion-resistant pump 6 and the grinding device 3 were started after stirring and reacting for 10 minutes, and the reaction was further carried out for 30 minutes to obtain lithium fluoride- The hydrofluoric acid mixed slurry was filtered, the filter residue was washed to PH~7, and 68.12g of lithium fluoride product was obtained after drying. The conversion rate of lithium carbonate was 97.0%, while the conversion rate of lithium carbonate by conventional stirring reaction was only 70%. %about. According to HG / T 4507-2013 "High Purity Industrial L...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com