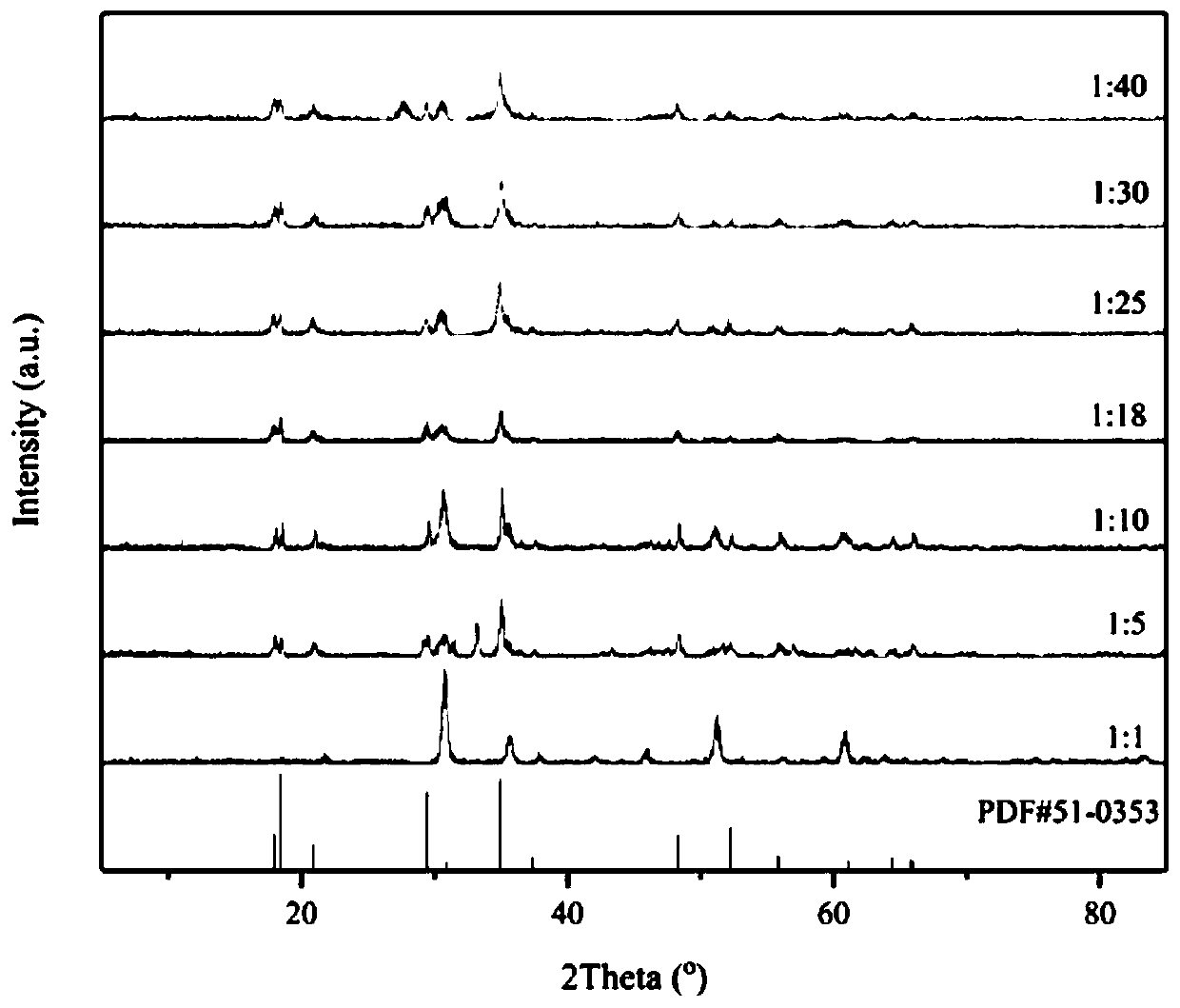
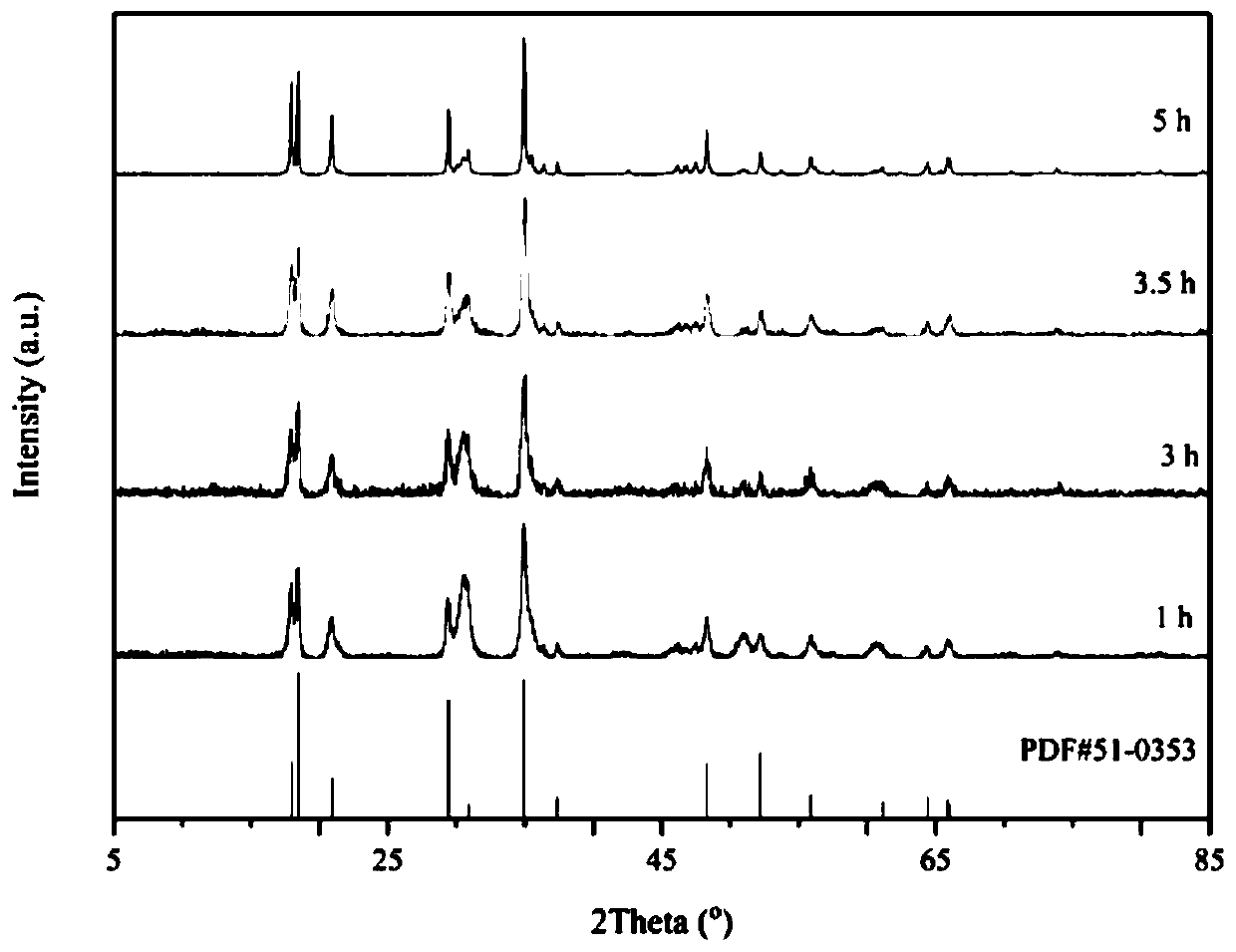
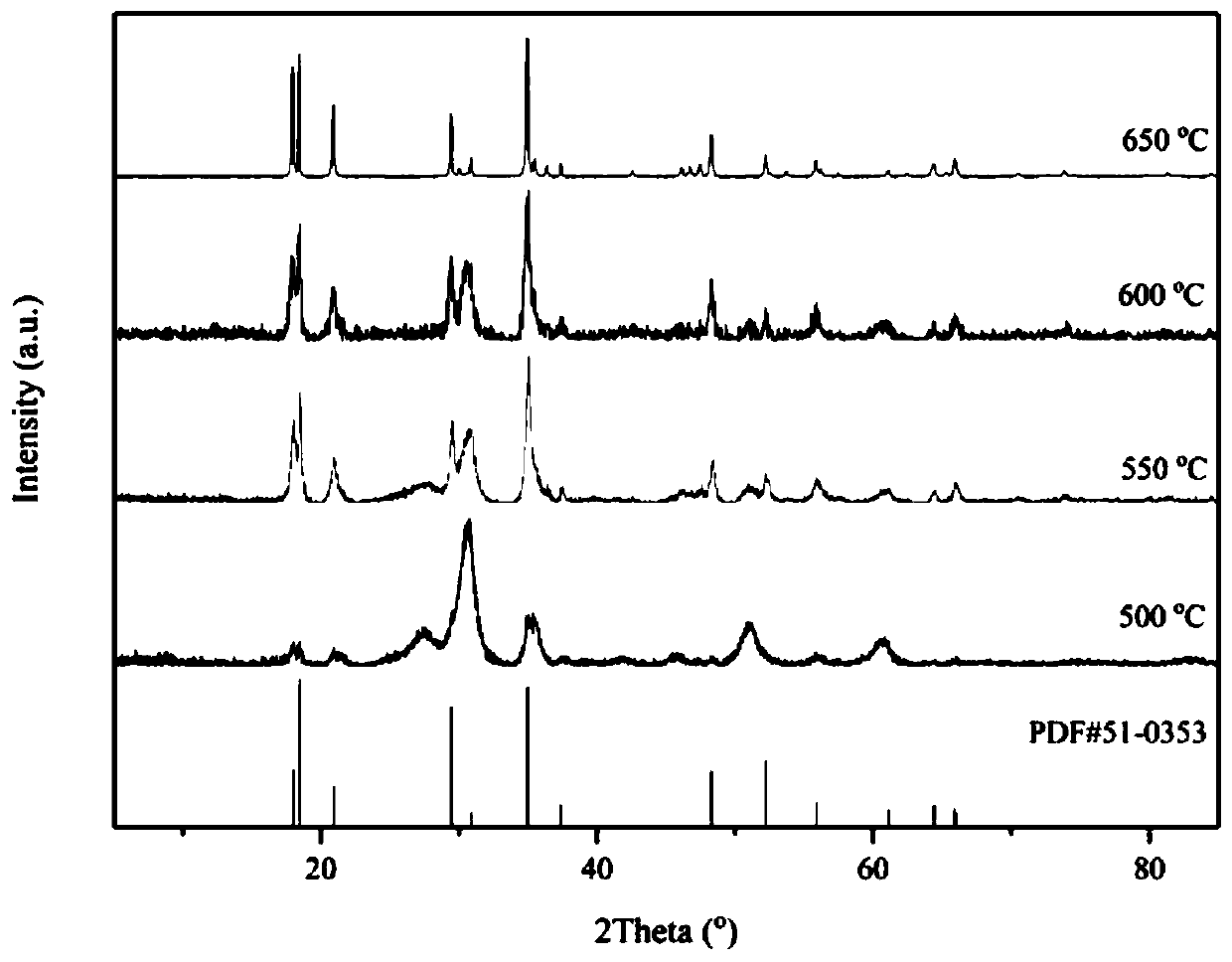
Preparation method of In<2.24>(NCN)3 powder
A powder and solid technology, applied in the field of preparation of In2.243 powder, can solve the problems of NaCN raw material being highly toxic, time-consuming, difficult to mass-produce, etc., and achieve low product cost, simple operation steps, and short preparation period. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] According to the In:N molar ratio of 1:1, 1.5041 g (5 mmol) of hydrated indium nitrate and 0.1051 g (0.83 mmol) of melamine were weighed and dissolved in 15 mL of methanol to make them evenly mixed, and then the solvent was evaporated to dryness by rotary evaporation at 50 °C. , the obtained solid mixture was calcined at 650 °C for 3 h in an argon atmosphere, and cooled to room temperature naturally to obtain In 2.24 (NCN) 3 powder.
Embodiment 2
[0032] According to the In:N molar ratio of 1:5, 1.5041g (5mmol) of hydrated indium nitrate and 0.5255g (4.2mmol) of melamine were weighed and dissolved in 15mL of methanol to make them evenly mixed, and then the solvent was evaporated to dryness by rotary evaporation at 50°C. , the obtained solid mixture was calcined at 650 °C for 3 h in an argon atmosphere, and cooled to room temperature naturally to obtain In 2.24 (NCN) 3 powder.
Embodiment 3
[0034] According to the In:N molar ratio of 1:10, 1.5041g (5mmol) of hydrated indium nitrate and 1.0510g (8.3mmol) of melamine were weighed and dissolved in 15mL of methanol to make them evenly mixed, and then the solvent was evaporated to dryness by rotary evaporation at 50°C. , the obtained solid mixture was calcined at 650 °C for 3 h in an argon atmosphere, and cooled to room temperature naturally to obtain In 2.24 (NCN) 3 powder.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com