Double-center cationic liquid for lithium battery and preparation method of cationic liquid
A technology of ionic liquid and cation, which is applied in the field of double-center cationic liquid and its preparation, can solve the problems of reduced charge and discharge performance of battery rate, achieve the effect of improving conductivity, improving cycle performance, and having no by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] (1) Dissolve bis(chloroethyl) ether (4.06 g, 28.4 mmol) and 1-methylpyrrole (5.13 g, 60.2 mmol) in 10 mL of distilled acetonitrile, and stir at reflux for 4 hours. The resulting mixture was cooled to room temperature and stirred overnight. Rotate to evaporate the solvent. After the product was washed three times with 15 ml of ether, the remaining solvent was removed under vacuum to obtain the desired compound 1,1'-(oxybis(ethyl-2 1-yl))dichlorobis( 1-methylpyrrolium-1-yl) salt (yield: 8.05 g, yield 90.5%) yellow liquid.
[0035] (2) Take the product 1,1'-(oxybis(ethyl-2 1-yl)) dichloride bis(1-methylpyrrolium-1-yl) salt (1.85g, 5.9mmol) obtained in the previous step ), dissolved in 8 ml of deionized water, and a slight excess of lithium bis(trifluoromethylsulfonyl)imide (LiTFSI, 3.55 g, 12.37 mmol) was added. The mixed solution was stirred overnight at room temperature to fully exchange halogen anions, and extracted with 30 ml of dichloromethane. To obtain an organic la...
Embodiment 2
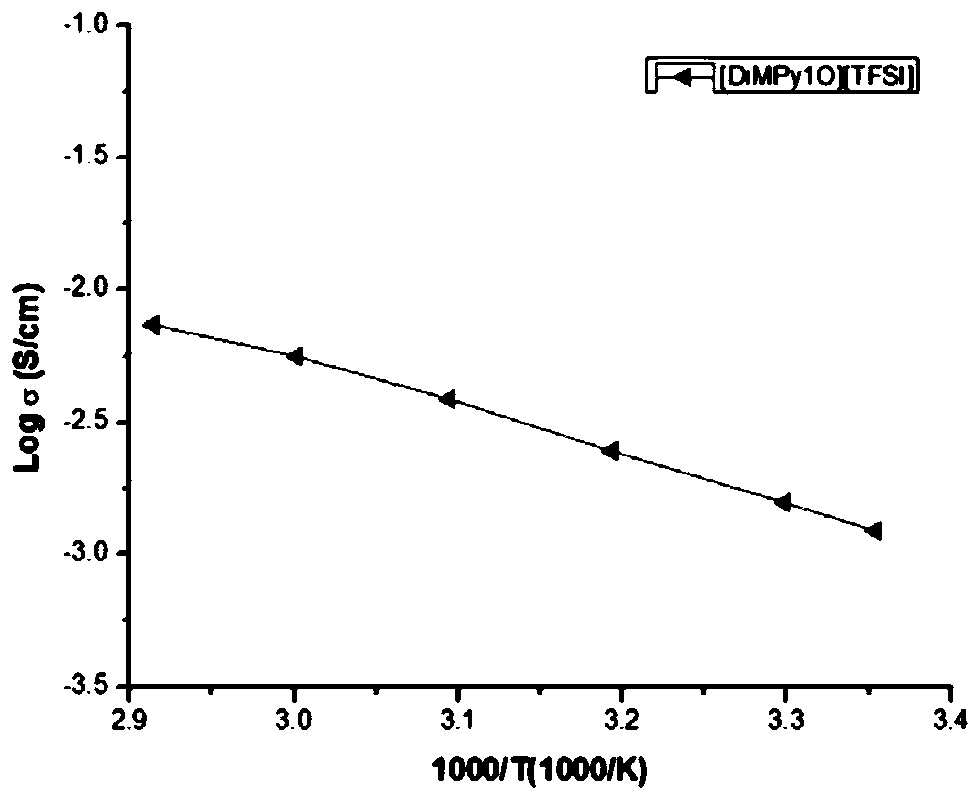
[0044] [DiMPy2O][TFSI] was prepared using the similar method in Example 1:
[0045] 1,2-bis(2-chloroethoxy)ethane (2.99g, 16mmol), 1-methylpyrrole (2.86g, 33.6mmol), the intermediate product (5.43g, yield 95.1%);
[0046] Take the intermediate product (4.08g, 11.4mmol) and LiTFSI (6.49g, 22.6mmol) to obtain the final product [DiMPy2O][TFSI] (6.49g, yield 67.1%);
[0047] The infrared spectrum of the product [DiMPy2O][TFSI]: 3563cm -1 (w),2964cm -1 ,2902.84(C-H,s),2379.03cm -1 ,1931.76-1798.58cm -1 (w),1634.43cm -1 ,1463.43cm -1 ,(s=o,s),1352.48cm -1 (C-N,w),1193.83cm -1 (C-O,s),1054.56cm -1 (C-F,s),953.83-877.41cm -1 ,789.69-739.92cm -1 .
[0048] 1 H NMR (300MHz, CDCl 3 ): δ (ppm) 3.84 (m, 4H), 3.73 - 3.50 (m, 16H), 3.03 (s, 6H), 2.17 (m, 8H). 13 C NMR nuclear magnetic carbon spectrum (300MHz, CDCl 3 ): δ (ppm) 124.02, 121.9, 117.7, 114.44, 70.2, 65.4, 65.0, 63.5, 48.6, 21.1.
[0049] First mix the ionic liquid synthesized in Example 2 with [MImEt][DCA] according to a weight ratio of 1...
Embodiment 3
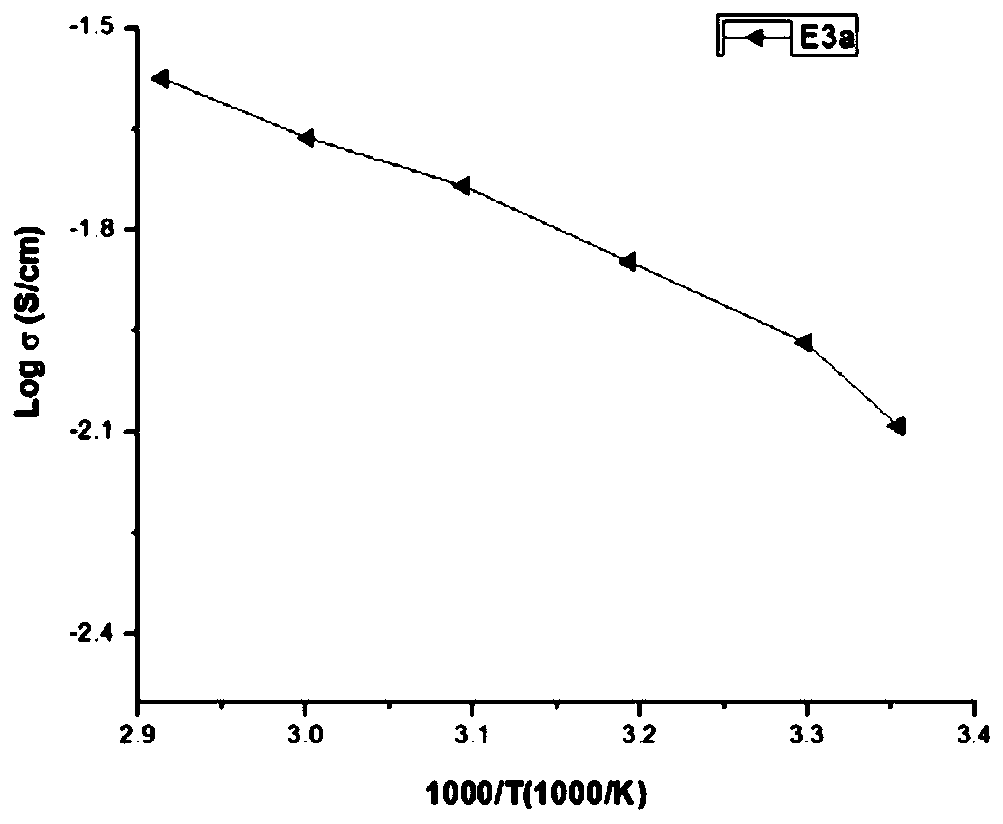
[0052] [DiMPy3O][TFSI] was prepared using the similar method in Example 1:
[0053] 1-chloro-2-(2-((2-chloroethoxy)ethoxy)ethoxy)ethane (4.01g, 17.3mmol), 1-methylpyrrole (3.14g, 36.9mmol), get Intermediate product (6.58g, yield 94.5%);
[0054] Take the intermediate product (2.01g, 5.0mmol) and LiTFSI (3.02g, 10.5mmol) to obtain the final product [DiMPy3O][TFSI] (3.17g, yield 71%);
[0055] The infrared spectrum of the product [DiMPy3O][TFSI]: 3563cm -1 (w), 2973cm -1 ,2877.73(C-H,s),2387.01cm -1 ,1931.61-1797.99cm -1 (w),1673.96cm -1 ,1462.84cm -1 ,(s=o,s),1352.42cm -1 (C-N,w),1193.55cm -1 (C-O,s),1056.58cm -1 (C-F,s),998.26-877.85cm -1 ,789.24-741.40cm -1 .
[0056] 1 H NMR proton nuclear magnetic spectrum ((300MHz, CDCl 3 ): δ (ppm) 3.87 (m, 4H), 3.75-3.54 (m, 20H), 3.08 (s, 6H), 2.21 (m, 8H). 13 C NMR nuclear magnetic carbon spectrum (300MHz, CDCl 3 ): δ (ppm) 124.02, 121.9, 117.7, 114.44, 70.63, 70.16, 65.50, 64.90, 48.69, 21.2.
[0057] First mix the ionic liquid synthesized in ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com