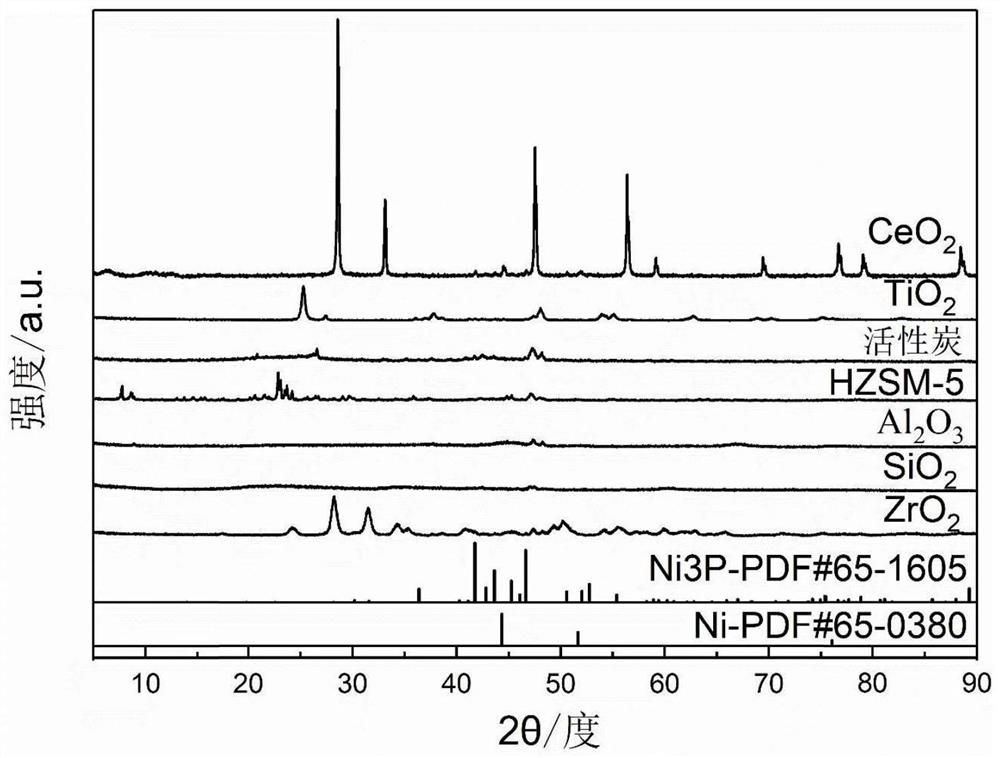
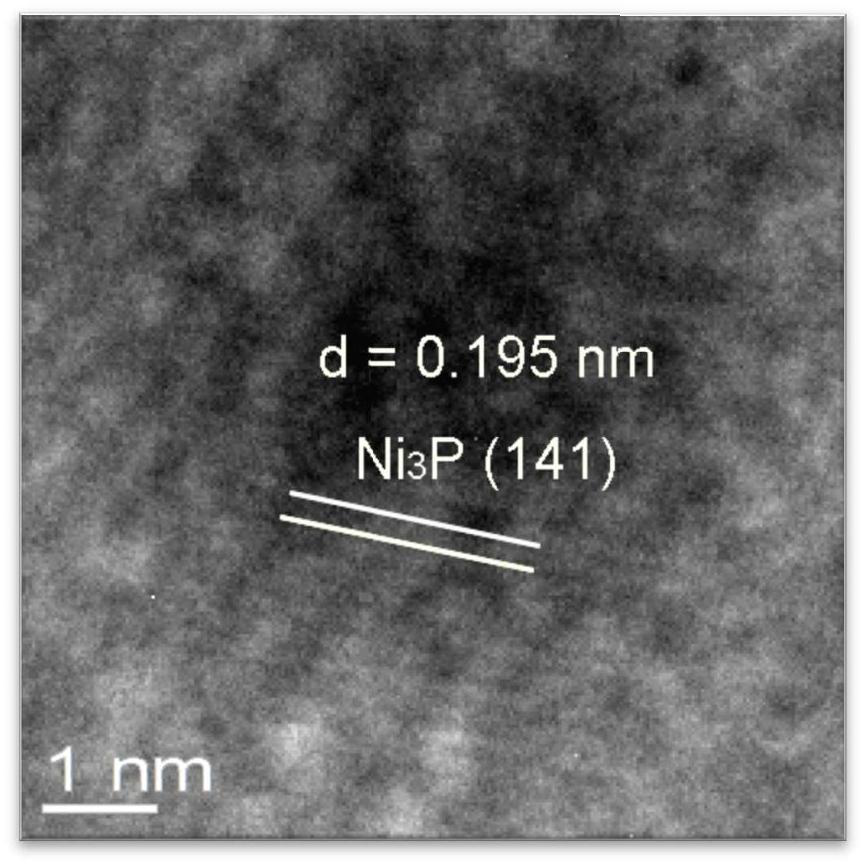
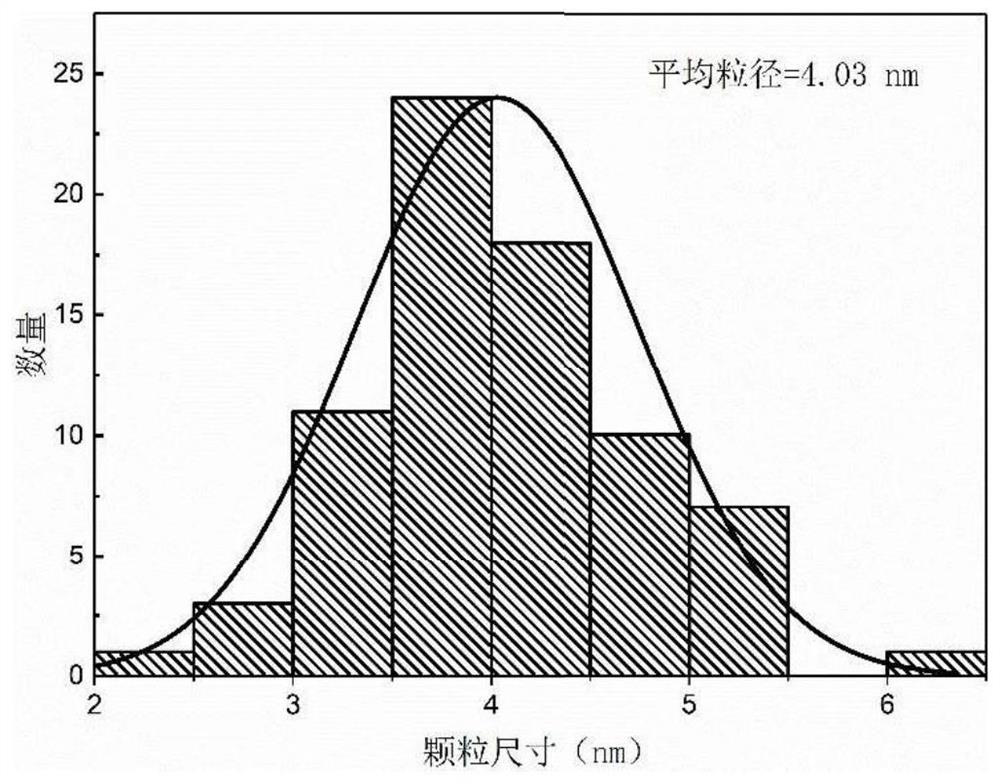
A kind of preparation method of highly dispersed supported nickel phosphide catalyst
A supported nickel phosphide technology, applied in the direction of molecular sieve catalysts, physical/chemical process catalysts, chemical instruments and methods, etc., can solve the problems of reduced activity, uneven dispersion, large catalyst particle size, etc., and achieve good stability, Effect of improving dispersion and good HDO activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The precursor was prepared by deposition and precipitation, and Ni was prepared by electroless plating. 3 P / Al 2 o 3 catalyst.
[0031] 2.6g Ni(NO 3 ) 2 ·6H 2 O was dissolved in 300 mL deionized water to make Ni(NO 3 ) 2 solution, add 2.4g Al to 240mL of this solution 2 o 3 carrier, heated to 70°C under constant stirring to form a suspension; weigh 7.6g of urea and add it to the remaining 60mL of Ni(NO 3 ) 2 Add 0.4mL of concentrated nitric acid to the solution, and add it dropwise to the above suspension at 70°C, raise the temperature to 90°C after dropping, and react for 16h. After the reaction is completed, filter with suction, wash with deionized water until the filtrate is neutral, and dry overnight in an oven at 110°C to obtain a gray-black solid, which is the precursor compound; then prepare 100 mL of acetic acid-sodium acetate buffer solution with a pH of 5.5, Then add 9.55g of sodium hypophosphite, heat up to 90°C under constant stirring, slowly add ...
Embodiment 2
[0034] 2.6g Ni(NO 3 ) 2 ·6H 2 O was dissolved in 300 mL deionized water to make Ni(NO 3 ) 2 solution, add 2.4g of HZSM-5 carrier to 240mL of the solution, and heat to 70°C under constant stirring to form a suspension; weigh 7.6g of urea and add it to the remaining 60mL of Ni(NO 3 ) 2 Add 0.4mL of concentrated nitric acid to the solution, and add it dropwise to the above suspension at 70°C, raise the temperature to 90°C after dropping, and react for 16h. After the reaction is completed, filter with suction, wash with deionized water until the filtrate is neutral, and dry overnight in an oven at 110°C to obtain a gray-black solid, which is the precursor compound; then prepare 100 mL of acetic acid-sodium acetate buffer solution with a pH of 5.5, Then add 9.55g of sodium hypophosphite, heat up to 90°C under constant stirring, slowly add 1.6g of precursor compound within 1h; after the reaction is completed, filter with suction, wash with deionized water until the filtrate is ...
Embodiment 3
[0037] 2.6g Ni(NO 3 ) 2 ·6H 2 O was dissolved in 300 mL deionized water to make Ni(NO 3 ) 2 solution, add 2.4g SiO to 240mL of this solution 2 carrier, heated to 70°C under constant stirring to form a suspension; weigh 7.6g of urea and add it to the remaining 60mL of Ni(NO 3 ) 2 Add 0.4mL of concentrated nitric acid to the solution, and add it dropwise to the above suspension at 70°C, raise the temperature to 90°C after dropping, and react for 16h. After the reaction is completed, filter with suction, wash with deionized water until the filtrate is neutral, and dry overnight in an oven at 110°C to obtain a gray-black solid, which is the precursor compound; then prepare 100 mL of acetic acid-sodium acetate buffer solution with a pH of 5.5, Then add 9.55g of sodium hypophosphite, heat up to 90°C under constant stirring, slowly add 1.6g of precursor compound within 1h; after the reaction is completed, filter with suction, wash with deionized water until the filtrate is neut...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com