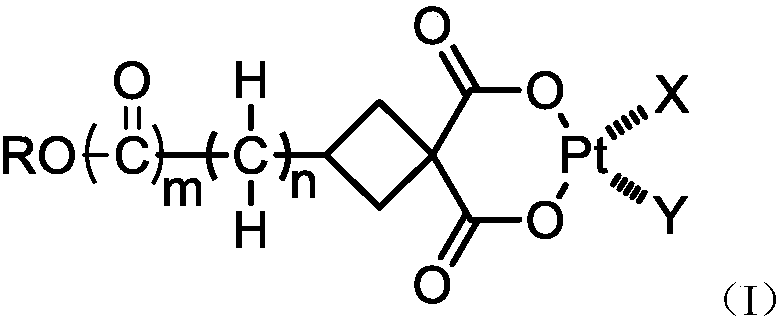
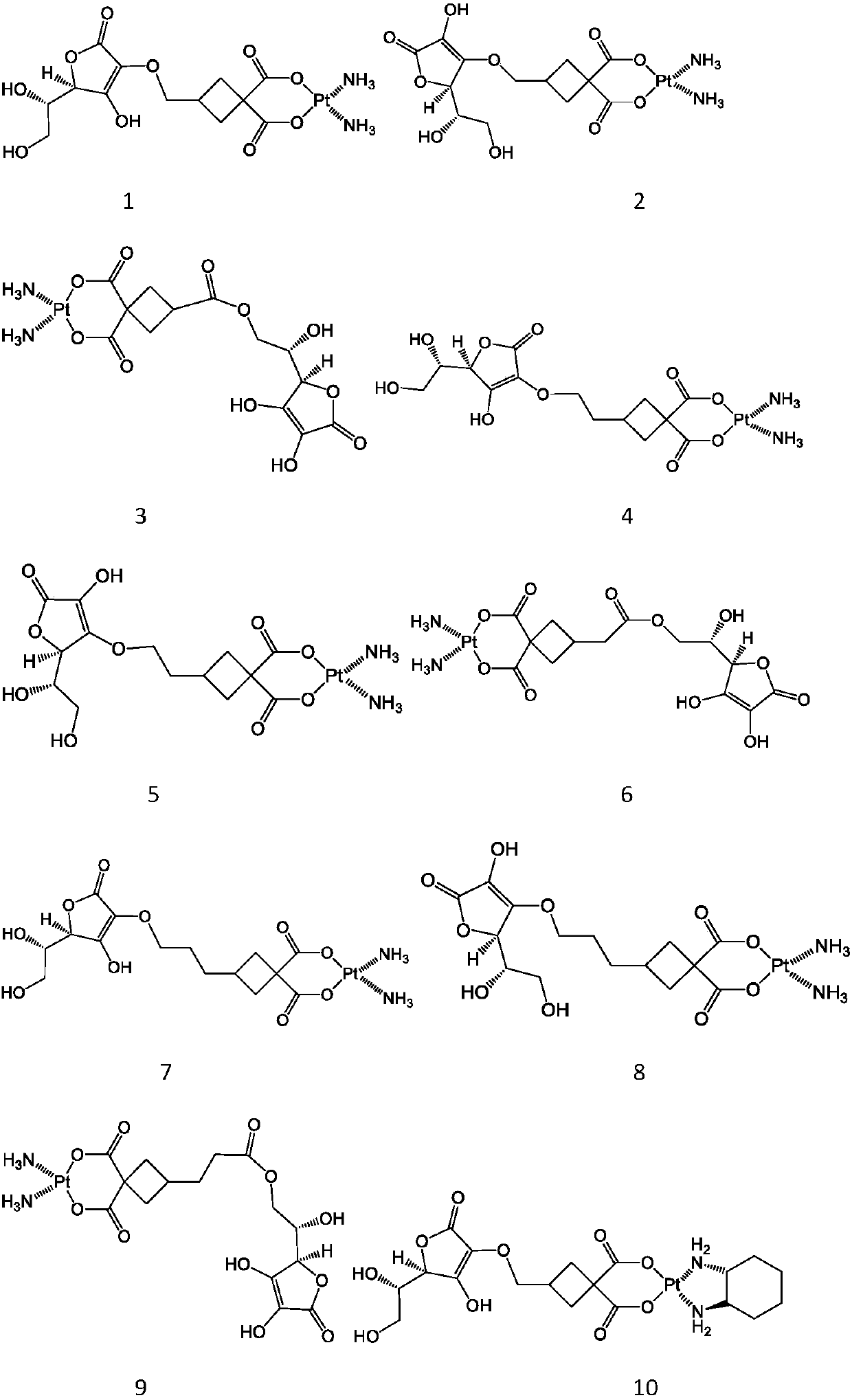
Vitamin C coupled platinum complex, intermediate thereof, preparation method thereof, pharmaceutical composition and application thereof
A platinum complex and vitamin technology, applied in the field of vitamin C coupled platinum complexes, can solve the problems of toxicity, side effects, drug resistance, low water solubility, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Preparation of diaminoplatinum(II) [3-methylene-(2-O-L-ascorbic acid) cyclobutane-1,1-dicarboxylic acid]
[0082]
[0083] (1) Preparation of 3-benzyloxymethyl-cyclobutane-1,1-di-tert-butyl dicarboxylate
[0084]
[0085] Sodium hydride (286 mg) was suspended in 5 mL of N,N-dimethylformamide, the air in the flask was replaced with nitrogen, and the flask was placed in an ice bath. Under the protection of nitrogen, slowly add di-tert-butyl malonate (1.6mL) dropwise, and after 0.5 hours of reaction, slowly add 2-benzyloxymethyl-1,3-dibromopropane (1.15g) in N,N -Dimethylformamide (5 mL) solution, the reaction solution was warmed up to 70°C and stirred for 7 hours. Monitor the end point of the reaction with TLC. After the reaction is complete, cool the reaction solution to room temperature, add 100 mL of ethyl acetate to the reaction solution, then wash with saturated aqueous ammonium chloride (1×50 mL), and extract the aqueous phase with ethyl acetate. (2 x 25 mL), ...
Embodiment 2
[0113] Preparation of diaminoplatinum(II) [3-methylene-(3-O-L-ascorbic acid) cyclobutane-1,1-dicarboxylic acid]
[0114]
[0115] (1) Preparation of 5,6-O-isopropylidene-3-O-(3-methylene-cyclobutane-1,1-di-tert-butyl dicarboxylate) L-ascorbic acid
[0116]
[0117] Dissolve 5,6-O-isopropylidene L-ascorbic acid (0.8g) in 5mL dimethyl sulfoxide at room temperature, add sodium bicarbonate (0.3g), and stir the reaction solution for 20 minutes, then add Di-tert-butyl 3-bromomethyl-cyclobutane-1,1-dicarboxylate (0.9 g) was dissolved in dimethyl sulfoxide (5 mL), the temperature of the reaction solution was raised to 60° C., and stirred overnight. Monitor the end point of the reaction with TLC. After the reaction is complete, cool the reaction solution to room temperature, add 50 mL of water to the reaction solution for dilution, neutralize with 1M dilute hydrochloric acid, then extract with 100 mL of ethyl acetate, and use distilled water (1 ×100mL), washed with saturated sod...
Embodiment 3
[0128] Preparation of diaminoplatinum(II) [3-formic acid-(6-O-L-ascorbic acid) cyclobutane-1,1-dicarboxylic acid]
[0129]
[0130] (1) Preparation of 3-formic acid-cyclobutane-1,1-di-tert-butyl dicarboxylate
[0131]
[0132] 3-Hydroxymethyl-cyclobutane-1,1-di-tert-butyl dicarboxylate (0.8 g) was dissolved in 10 mL of acetone, and Jones reagent (chromium trioxide 420 mg, concentrated sulfuric acid 371 μL , diluted with water to 1.6mL), the reaction solution was slowly warmed up to room temperature, stirred for 2 hours, and the reaction was monitored by TLC. After the reaction was completed, a few drops of isopropanol were added dropwise to remove excess oxidant. The reaction solution was filtered with suction, and the solvent was evaporated to dryness with a rotary evaporator. The obtained green oil was diluted with water, extracted with ethyl acetate, the organic phase was washed with saturated sodium chloride solution, dried with anhydrous sodium sulfate, and the solv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com