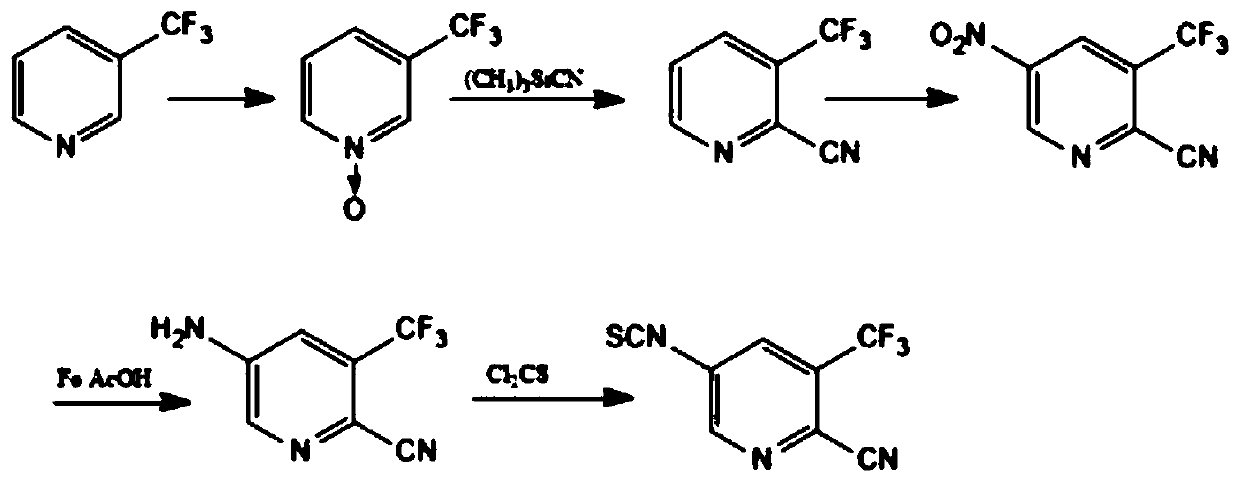
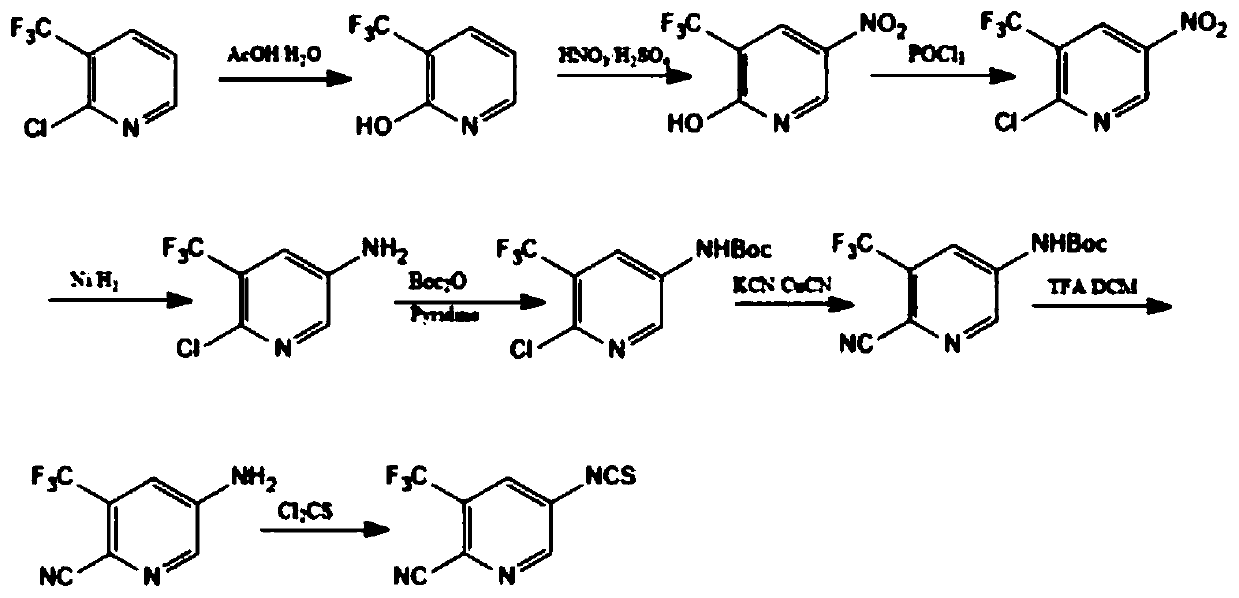
Preparation method of 5-isothiocyanato-3-(trifluoromethyl)pyridine-2-cyano
A technology of isothiocyanate and trifluoromethyl is applied in the field of preparation of 5-isothiocyanate-3-pyridine-2-cyano, which can solve the problems of low total yield, long reaction steps, and low yield. problem, to achieve the effect of high yield, low cost and easy industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
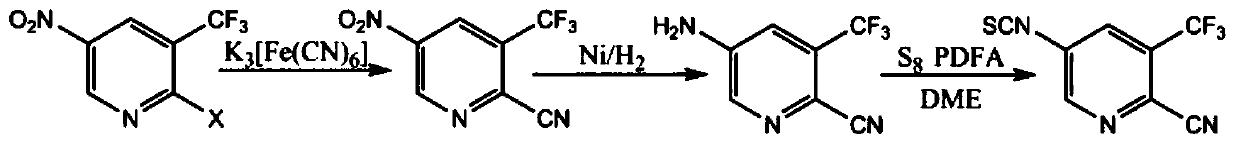
[0032] A preparation method of 5-isothiocyanato-3-(trifluoromethyl)pyridine-2-cyano group, is characterized in that, comprises the following steps:
[0033] Step 1: Add 106g sodium carbonate, 0.1g palladium acetate, and 1.5L DMF to the reaction flask, stir, add 0.2gdppf (1,1-diphenylphosphinoferrocene), after reflux, add 271g 2-bromo- 3-trifluoromethyl-5-nitropyridine, add 100g potassium ferrocyanide, nitrogen protection, reflux reaction for 20 hours, after the reaction, quench with water, extract with n-hexane, wash with brine, dry, and distill under reduced pressure Solvent, to obtain 206g of intermediate 1, yield: 95%.
[0034] Step 2: add 200g of intermediate 1 to the autoclave, then add Raney nickel, 650mL of ethanol, and feed hydrogen to 2 atm. After the reaction is completed, cool, replace with nitrogen, filter, wash, and concentrate the filtrate to obtain 155g of intermediate 2. Yield: 90%.
[0035] Step 3: Add 150g of intermediate 2 to the reaction flask, then add 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com