Method for improving solid-state battery interface stability by polymer electrolyte
A polymer, solid electrolyte technology, applied in the field of lithium germanium phosphate, lithium ion batteries, can solve the problems of low load of electrode active material, volume change of electrode material, poor cycle stability, etc., to avoid lithium loss, high air stability, The effect of improving compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
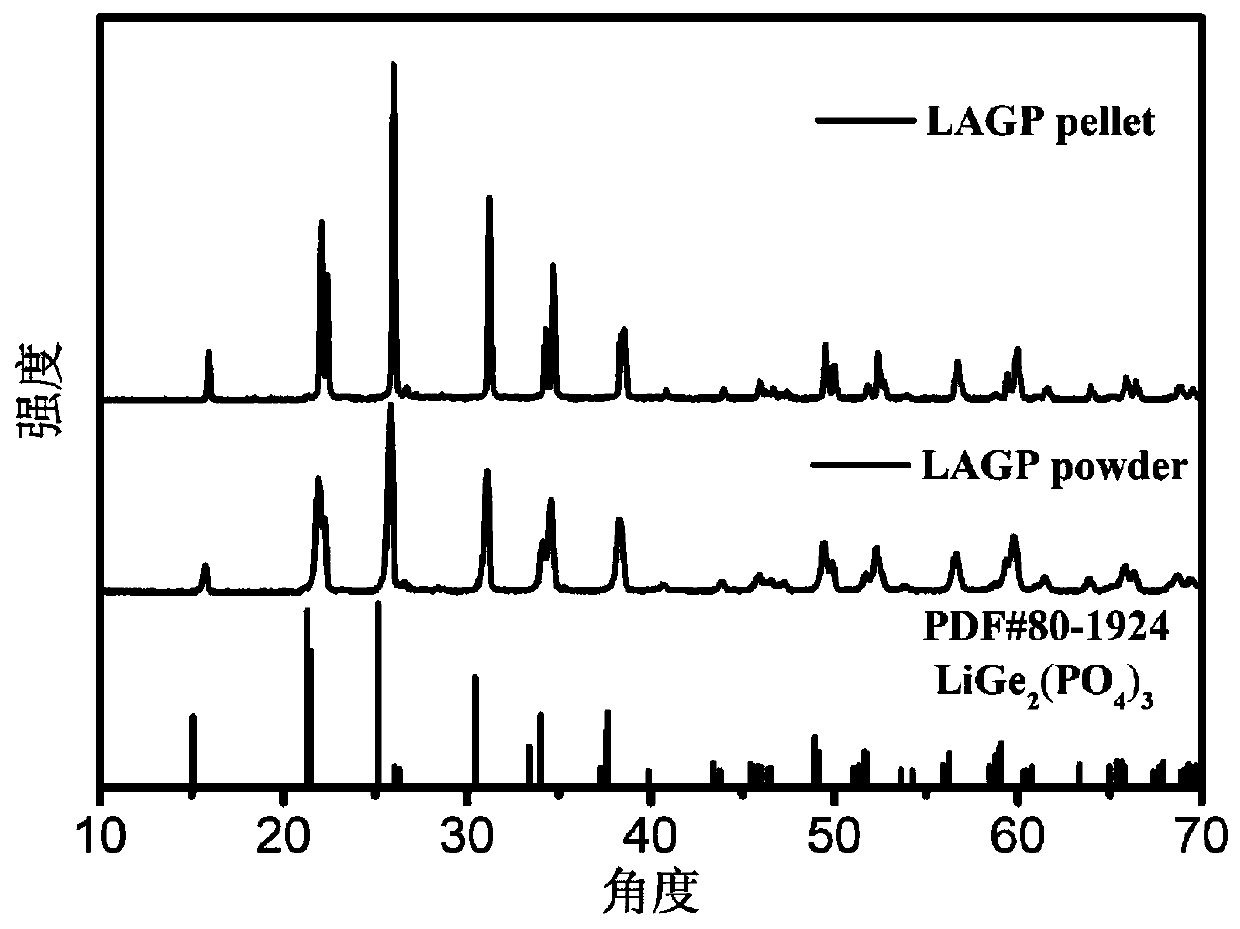
[0040] According to the characteristics of the aqueous solution method, lithium hydroxide monohydrate, aluminum hydroxide, germanium dioxide, and ammonium dihydrogen phosphate are preferably used as raw materials for preparing LAGP. According to Li 1.5 Al 0.5 Ge 1.5 (PO 4 ) 3 Stoichiometric molar ratio Weigh lithium hydroxide monohydrate (5% to 15% in excess), aluminum hydroxide, germanium dioxide, ammonium dihydrogen phosphate (2% in excess), dissolve in water and stir evenly with a magnetic stirrer. After the dispersion is uniform, a certain amount of 28% dilute ammonia water is added dropwise to the mixed liquid to adjust the pH to a strong alkalinity (above 12). After the reaction is complete, the solvent is evaporated by heating in a sand bath and completely dried to obtain crystals. As the solvent evaporated, white crystals were obtained. Fully grind to obtain a white powder, place it in an alumina porcelain boat, and dry it in a blast drying oven for 6 hours to fur...
Embodiment 2
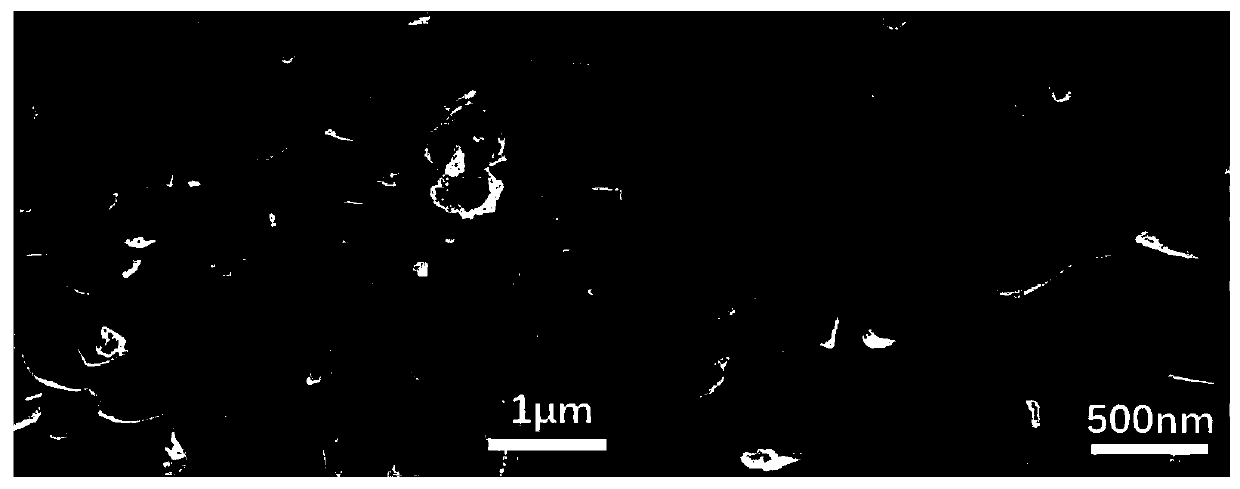
[0042] The morphology and particle size distribution of the solid electrolyte material were observed under a scanning electron microscope. figure 2 The experimental results given show that the particle size distribution is uniform, and the particles are smaller and more uniform than the solid-phase method.
Embodiment 3
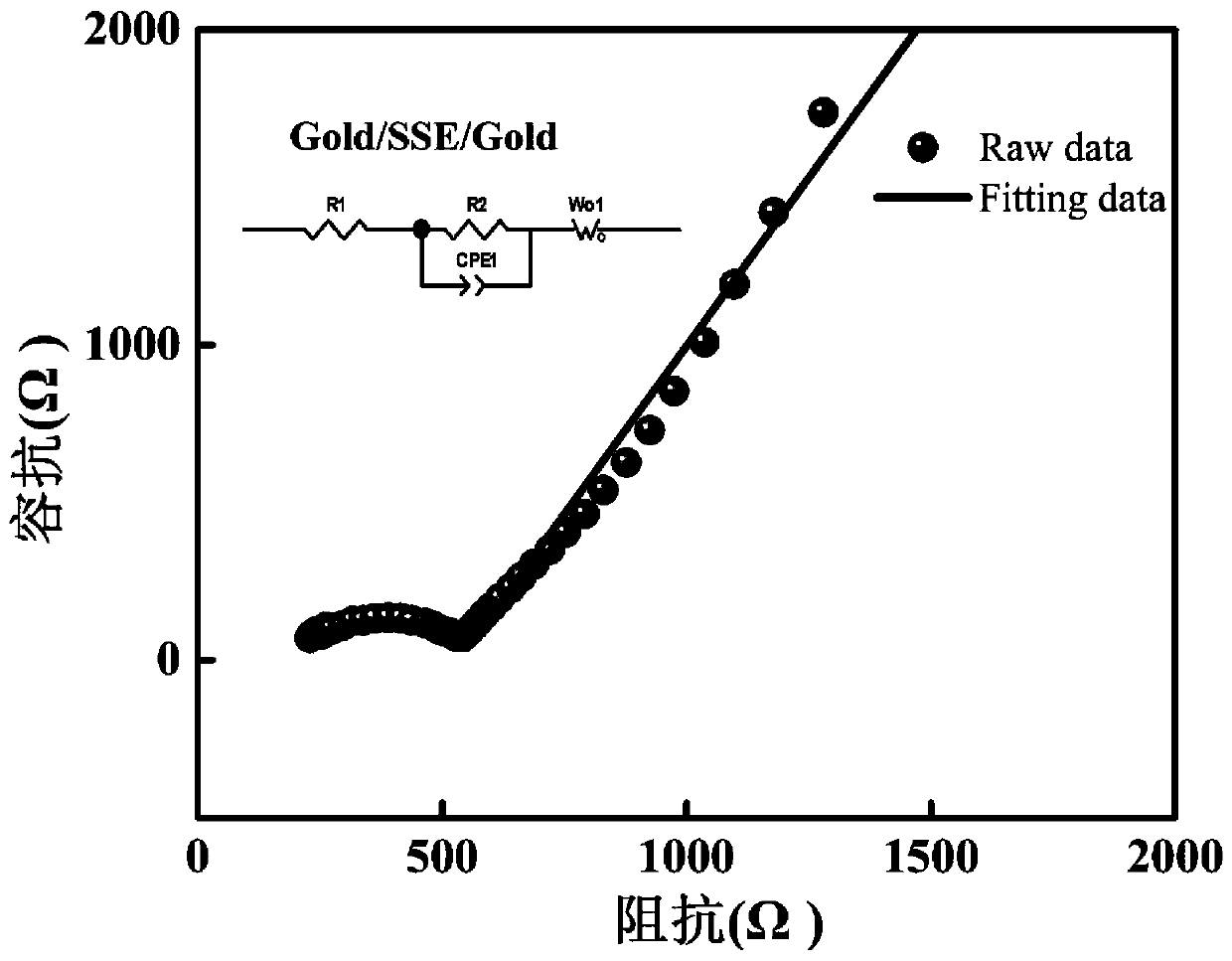
[0044] The conductivity test of inorganic solid electrolytes is generally carried out in the form of a blocking cell, and metals such as Au or Ag are used as blocking electrodes on both ends of the ceramic sheet for testing, because Au has a negative effect on Li + It has a certain blocking effect, so it presents an obvious capacitive reactance arc in the low frequency region of the impedance spectrum. The prepared ceramic sheets were polished with different sandpapers, and after the surface of the LAGP ceramic sheets was plated with gold, the AC impedance test was carried out on the LAGP ceramic sheets. Using EIS AC impedance spectroscopy for testing. The frequency range is 10Hz ~ 1MHz, and the test temperature is room temperature. image 3 is the AC impedance spectrum of the LAGP ceramic sheet, according to the formula (d: thickness of ceramic sheet, cm; R: total impedance of ceramic sheet, Ω; S: cross-sectional area of ceramic sheet, cm -2 ) can calculate its ion cond...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com