A kind of paliperidone tablet and preparation method thereof
A technology for paliperidone and sustained-release tablets, which is applied in the field of medicine and can solve the problems of uneven content of small doses of drugs, changes in paliperidone, and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The present invention also provides a preparation method of the paliperidone press-coated sustained-release tablet described in the above technical scheme, comprising the following steps:
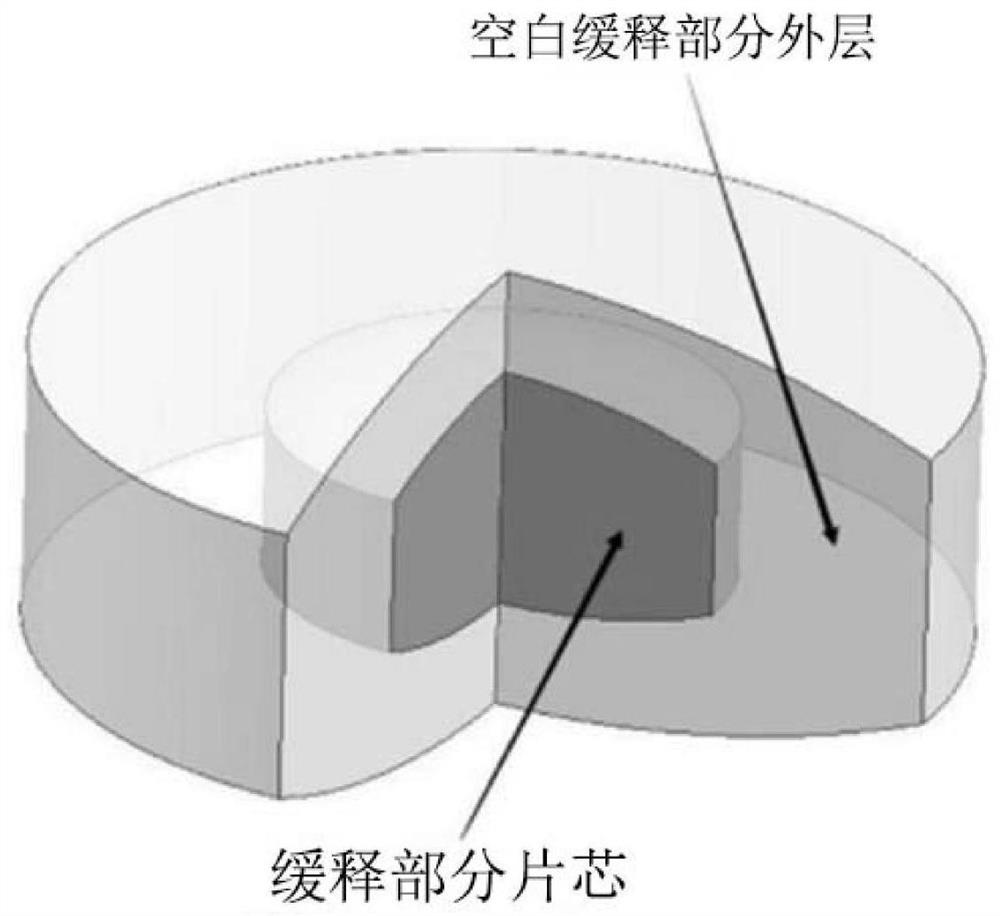
[0045] 1) mixing the filler and retarder, and wet granulating the binder solution to obtain the first granular material; mixing the first granular material with paliperidone, lubricant and slow-release material to obtain the mixed material, Compress to obtain the sustained-release portion of the tablet core;
[0046] 2) Mix the filler, blocker and microenvironment regulator, and use the binder solution to wet granulate to obtain the second granular material; mix the second granular material with the lubricant and the slow-release material to obtain the blank slow-release part of the outer layer;
[0047] 3) filling the outer layer of the blank sustained-release part, the tablet core of the sustained-release part and the outer layer of the blank sustained-release part in sequence, an...
Embodiment 1
[0057] Paliperidone Sustained Release Tablets 3mg System (based on 1000 tablets)
[0058] The composition of the prescription is shown in Table 1.
[0059] Table 1 Paliperidone compression-coated sustained-release tablet (3mg) prescription composition table
[0060]
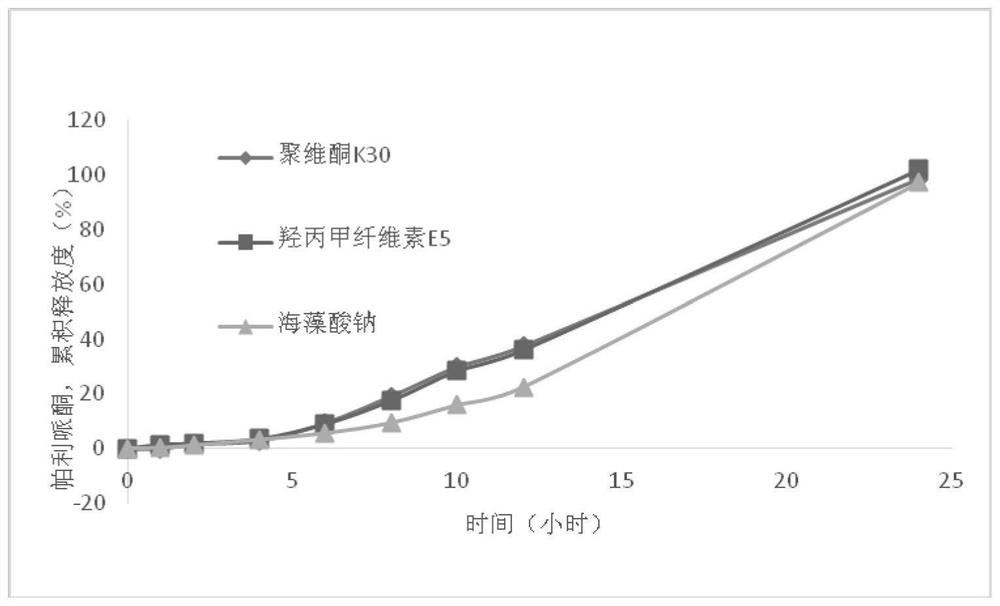
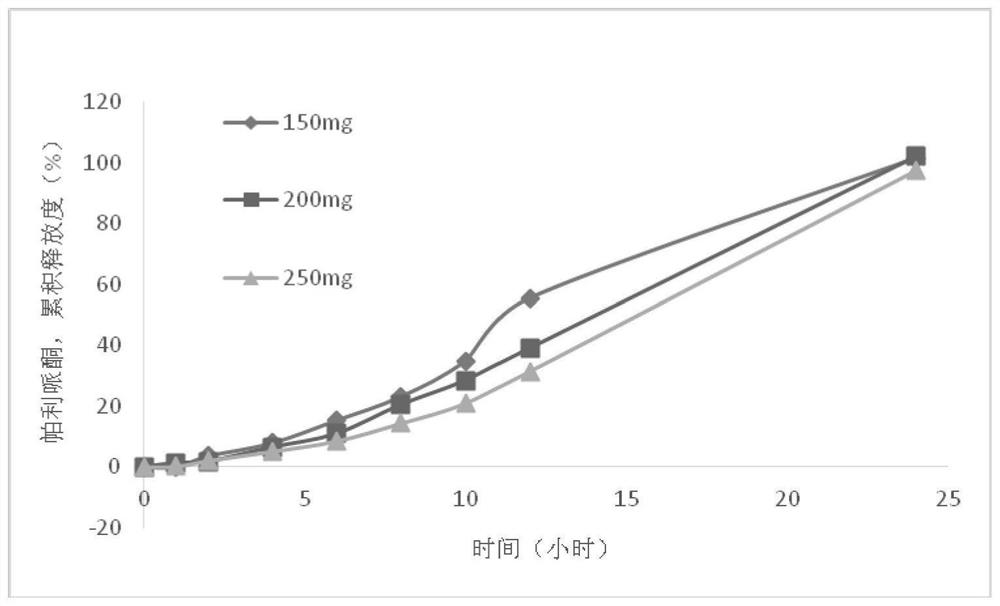
[0061] 1. Tablet core preparation: weigh Eudragit RLPO and microcrystalline cellulose according to the prescription amount, mix them evenly, make a soft material with 3% sodium alginate solution, pass through a 40-mesh sieve, extrude and granulate, and dry at 40°C After 2 hours, pass through a 40-mesh sieve for granulation, add hydroxypropyl cellulose, glyceryl behenate and paliperidone, mix well, and press a 5mm flat punch into a tablet. The tablet hardness is controlled at about 40N, and the mass range is 50±2.25 mg.
[0062] 2. Preparation of the pressing coating layer: Weigh Eudragit RLPO, microcrystalline cellulose and citric acid according to the prescription, mix well, use 3% sodium alginate solution ...
Embodiment 2
[0071] Paliperidone Sustained Release Tablets 6mg System (based on 1000 tablets)
[0072] The composition of the prescription is shown in Table 3.
[0073] Table 3 Paliperidone compression-coated sustained-release tablet (6mg) prescription composition table
[0074]
[0075] 1. Tablet core preparation: Weigh Eudragit RL PO and microcrystalline cellulose according to the prescription amount, mix well, make soft material with 3% sodium alginate solution, pass through a 40-mesh sieve, extrude and granulate, and store at 40°C Dry for 2 hours, pass through a 40-mesh sieve for granulation, add hydroxypropyl cellulose, glyceryl behenate and paliperidone, mix evenly, use a 5mm flat punch to make tablets, the tablet hardness is controlled at about 40N, and the mass range is 50± 2.25 mg.
[0076] 2. Preparation of the pressing coating layer: weigh Eudragit RL PO, microcrystalline cellulose and citric acid according to the prescription amount, mix well, use 3% sodium alginate soluti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com