Visible light photo-controlled acid-resistant fluorescent molecular switch and synthesis method thereof
A technology of fluorescent molecules and synthesis methods, applied in the field of acid-resistant fluorescent molecular switches and their synthesis, can solve the problems of failure of light-controlled fluorescent molecular switches, weak absorption, interference of fluorescent signals, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
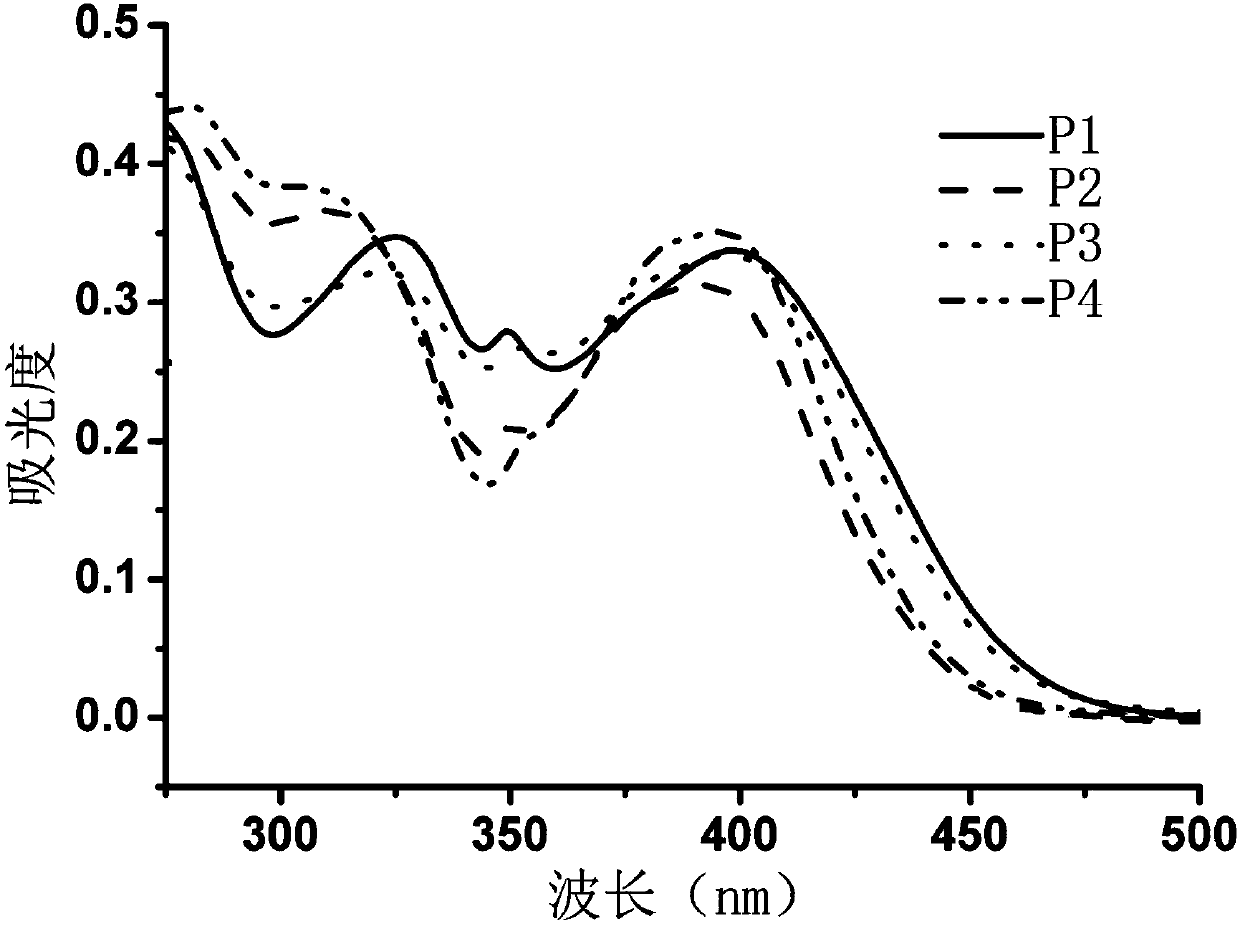
[0038] When R 1 = R 2 = R 3 = R 4 =C 2 h 5 ,R 5 =H, X=O, Y=H, when Z=O, its molecular (P1) synthesis route and product structure are as follows:
[0039]
[0040] Synthesis steps and characterization: 3-Nitrorhodamine (2.92g, 6mmol) and phosphorus oxychloride (5.6mL, 60mmol) were placed in 1,2-dichloroethane (150mL), heated to 84°C under reflux, stirred After 2 hours the solvent was evaporated to give a dark purple oily liquid. The crude acid chloride product was dissolved in dichloromethane (100mL), then added dropwise to a mixed solution of triethylamine (3mL) and 6-(4-aminophenylethynyl)naphthalene anhydride (1.88g, 6mmol), and stirred at room temperature for 24 hours Afterwards, the solvent was evaporated under reduced pressure, and the residue was separated by column chromatography (silica gel, dichloromethane / ethyl acetate, 30:1 v / v) to obtain a yellow powdery intermediate (2.44 g, 52%). Take the above yellow powder (1.56g, 2mmol), stannous chloride dihydrate ...
Embodiment 2
[0048] When R 1 = R 2 = R 3 = R 4 =C 2 h 5 ,R 5 =CH 3 CO, X=O, Y=H, when Z=O, its molecular (P2) synthesis route and product structure are as follows:
[0049]
[0050] Synthesis steps and characterization: P1 (0.75g, 1mmol) and acetyl chloride (0.12g, 1.5mmol) were mixed in dichloromethane (10mL), stirred for 2 hours, and the solvent was evaporated under reduced pressure, and the crude product was passed through column chromatography (silica gel, Ethyl acetate / petroleum ether, 1:3 v / v) isolated the product P2 as a yellow powder (0.76 g, 96%).
[0051] The product was characterized by NMR and mass spectrometry:
[0052] 1 H NMR (400MHz, CDCl 3 )δ10.58(s,1H),8.75(d,J=8.2Hz,1H),8.65(d,J=7.2Hz,1H),8.55(d,J=7.7Hz,1H),8.51(d, J=8.2Hz,1H),7.92(d,J=7.7Hz,1H),7.90–7.82(m,1H),7.56–7.43(m,3H),7.00(d,J=8.5Hz,2H), 6.81(d, J=7.6Hz, 1H), 6.67(d, J=8.8Hz, 2H), 6.37–6.26(m, 4H), 3.33(q, J=7.0Hz, 8H), 2.31(s, 3H ), 1.17 (t, J=7.0Hz, 12H). 13 CNMR (101MHz, CDCl 3)δ169.31,168.9...
Embodiment 3
[0058] When R 1 = R 2 = R 3 = R 4 =C 2 h 5 , R 5 =H, X=O, Y=H, Z=C 6 h 12 N 2 During 0, its molecular (P3) synthesis route and product structure are as follows:
[0059]
[0060] Synthesis steps and characterization: P1 (0.37g, 0.5mmol) and 2-ethylaminomorpholine (0.19g, 1.5mmol) were mixed in absolute ethanol (10mL), heated to 78°C and refluxed, stirred for 10 hours and then decompressed The solvent was evaporated, and the residue was separated by column chromatography (silica gel, ethyl acetate / petroleum ether, 1:2 v / v) to obtain a yellow powder product P3 (0.36 g, 84%).
[0061] The product was characterized by NMR and mass spectrometry:
[0062] 1 H NMR (400MHz, CDCl 3 )δ8.66(d, J=8.3Hz, 1H), 8.61(d, J=7.1Hz, 1H), 8.52(d, J=7.7Hz, 1H), 7.87(d, J=7.6Hz, 1H) ,7.79(t,J=7.8Hz,1H),7.44(d,J=8.4Hz,2H),7.22(t,J=7.7Hz,1H),7.10(d,J=8.5Hz,2H),6.76 (d, J=8.6Hz, 2H), 6.60(d, J=8.0Hz, 1H), 6.37(d, J=7.4Hz, 1H), 6.32(d, J=10.4Hz, 4H), 5.44(s ,2H),4.34(t,J=6.8Hz,2H),3.68...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com