A kind of boron-containing preparation with cell nucleus targeting and its preparation method and application
A targeting, cell nucleus technology, applied in the field of biomedicine, can solve the problem of poor cell nucleus targeting ability, achieve good biocompatibility, improve therapeutic effect, enhance in vivo application stability and target tissue tropism.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
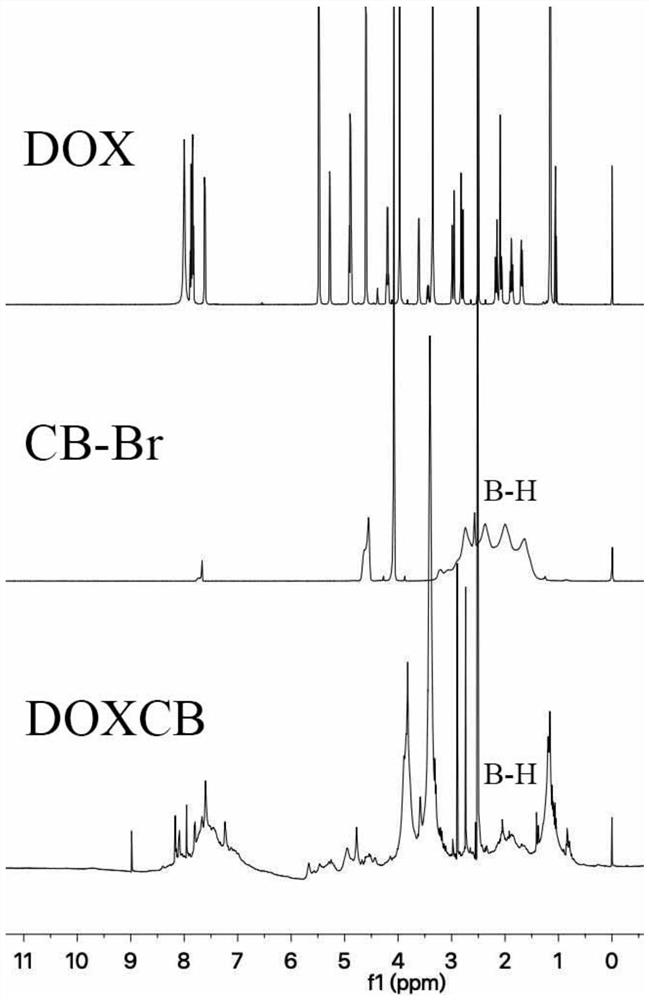
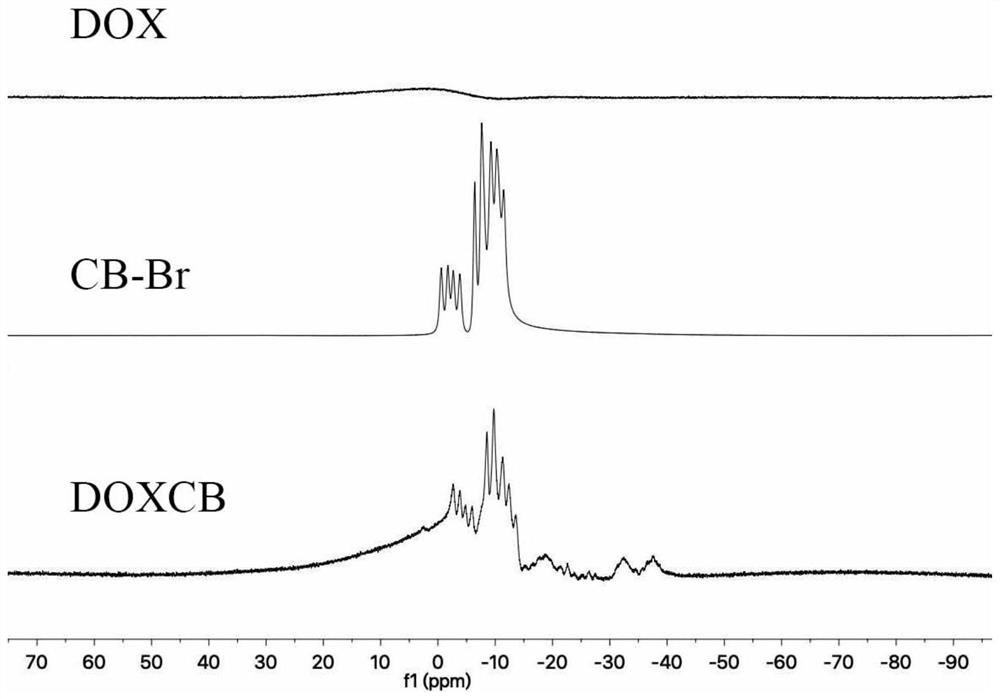
[0084] Embodiment 1 boron-containing doxorubicin preparation (DOXCB)
[0085] (A-1) Dissolve doxorubicin (i.e. doxorubicin hydrochloride) in anhydrous dimethyl sulfoxide, add triethylamine under nitrogen protection condition, stir for 2h, add 1-bromomethyl-o-carborane , magnetic sub-stirring at room temperature for 48h, then add excess ether, so that the precipitation is complete; the molar ratio of doxorubicin, triethylamine and 1-bromomethyl-o-carborane is 1:2:1;
[0086] (A-2) Collect the precipitate into a dialysis bag with a molecular weight cut-off of 500, use dimethyl sulfoxide as the dialysate for 24 hours, then use distilled water as the dialysate for 48 hours, and freeze-dry under reduced pressure to obtain a red powder, which is the Doxorubicin Borate (DOXCB).
[0087] The boron content of DOXCB in this embodiment is 5.15wt% as detected by inductively coupled plasma mass spectrometry (ICP-MS) after being digested with concentrated nitric acid.
[0088] Both DOX an...
Embodiment 2
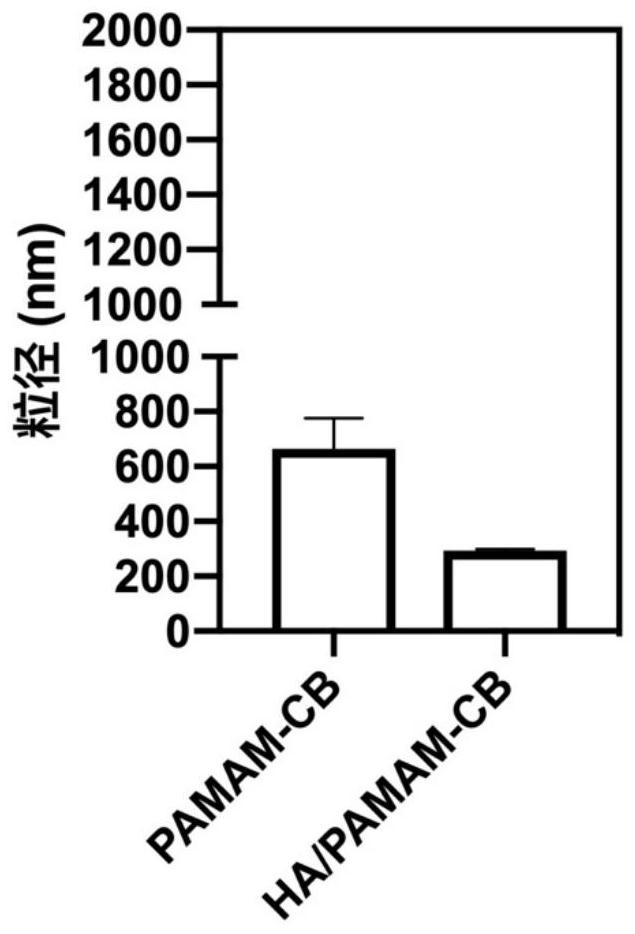
[0090] Example 2 Nano boron capture preparation (HA / PAMAM-CB)
[0091] (B-1) Add the 2nd generation dendritic polyamide, 1-bromomethyl-o-carborane and triethylamine into dimethyl sulfoxide in a molar ratio of 1:1:12, and after magnetic stirring at room temperature for 48 hours, add Excess ether until the precipitation is complete;
[0092] (B-2) Collect the precipitate into a dialysis bag with a molecular weight cut-off of 1000, use distilled water as the dialysate for 72 hours, then use a 200W power probe to sonicate for 3 minutes, pass through a 0.22 μm filter membrane, and freeze-dry the filtrate to obtain a dendritic high Molecular boron capture agent PAMAM-CB;
[0093] (B-3) Dissolve the dendrimer boron capture agent in water, add an equal mass of hyaluronic acid, let it stand for 0.5h, then ultrasonicate for 0.5h in a water bath, then ultrasonicate with a 200W power probe for 2min to obtain a clear aqueous solution, and obtain a clear HA / PAMAM - CB aqueous solution.
...
Embodiment 3
[0098] Embodiment 3 Dendrimer boron capture agent (PAMAM-BPABOC)
[0099] (C-1) Dissolve di-tert-butyl dicarbonate and 4-boron-L-phenylalanine in a molar ratio of 1:1 in a mixed solvent of tetrahydrofuran and distilled water with a volume ratio of 1:1, stir for 24 hours, and rotavap Remove tetrahydrofuran to obtain an aqueous solution containing the product, add 0.1M aqueous sodium bicarbonate solution to adjust the pH to 9, extract with ethyl acetate, and remove ethyl acetate by rotary evaporation to obtain product I;
[0100] (C-2) Dissolve the product I in distilled water, adjust the pH to 5 with 1M aqueous hydrochloric acid, add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide and N-hydroxysuccinyl imine, after stirring for 4h, add 1M sodium hydroxide aqueous solution to adjust the pH to 8, add 2 generations of dendritic polyamide, and continue stirring for 48h; the product I, 1-(3-dimethylaminopropyl)-3- The molar ratio of ethyl carbodiimide, N-hydroxysuccinimide and 2nd ge...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com